Internet Therapy for Abdominal Pain in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excesss Karaoke Master by Artist
XS Master by ARTIST Artist Song Title Artist Song Title (hed) Planet Earth Bartender TOOTIMETOOTIMETOOTIM ? & The Mysterians 96 Tears E 10 Years Beautiful UGH! Wasteland 1999 Man United Squad Lift It High (All About 10,000 Maniacs Candy Everybody Wants Belief) More Than This 2 Chainz Bigger Than You (feat. Drake & Quavo) [clean] Trouble Me I'm Different 100 Proof Aged In Soul Somebody's Been Sleeping I'm Different (explicit) 10cc Donna 2 Chainz & Chris Brown Countdown Dreadlock Holiday 2 Chainz & Kendrick Fuckin' Problems I'm Mandy Fly Me Lamar I'm Not In Love 2 Chainz & Pharrell Feds Watching (explicit) Rubber Bullets 2 Chainz feat Drake No Lie (explicit) Things We Do For Love, 2 Chainz feat Kanye West Birthday Song (explicit) The 2 Evisa Oh La La La Wall Street Shuffle 2 Live Crew Do Wah Diddy Diddy 112 Dance With Me Me So Horny It's Over Now We Want Some Pussy Peaches & Cream 2 Pac California Love U Already Know Changes 112 feat Mase Puff Daddy Only You & Notorious B.I.G. Dear Mama 12 Gauge Dunkie Butt I Get Around 12 Stones We Are One Thugz Mansion 1910 Fruitgum Co. Simon Says Until The End Of Time 1975, The Chocolate 2 Pistols & Ray J You Know Me City, The 2 Pistols & T-Pain & Tay She Got It Dizm Girls (clean) 2 Unlimited No Limits If You're Too Shy (Let Me Know) 20 Fingers Short Dick Man If You're Too Shy (Let Me 21 Savage & Offset &Metro Ghostface Killers Know) Boomin & Travis Scott It's Not Living (If It's Not 21st Century Girls 21st Century Girls With You 2am Club Too Fucked Up To Call It's Not Living (If It's Not 2AM Club Not -

Songs by Artist
Reil Entertainment Songs by Artist Karaoke by Artist Title Title &, Caitlin Will 12 Gauge Address In The Stars Dunkie Butt 10 Cc 12 Stones Donna We Are One Dreadlock Holiday 19 Somethin' Im Mandy Fly Me Mark Wills I'm Not In Love 1910 Fruitgum Co Rubber Bullets 1, 2, 3 Redlight Things We Do For Love Simon Says Wall Street Shuffle 1910 Fruitgum Co. 10 Years 1,2,3 Redlight Through The Iris Simon Says Wasteland 1975 10, 000 Maniacs Chocolate These Are The Days City 10,000 Maniacs Love Me Because Of The Night Sex... Because The Night Sex.... More Than This Sound These Are The Days The Sound Trouble Me UGH! 10,000 Maniacs Wvocal 1975, The Because The Night Chocolate 100 Proof Aged In Soul Sex Somebody's Been Sleeping The City 10Cc 1Barenaked Ladies Dreadlock Holiday Be My Yoko Ono I'm Not In Love Brian Wilson (2000 Version) We Do For Love Call And Answer 11) Enid OS Get In Line (Duet Version) 112 Get In Line (Solo Version) Come See Me It's All Been Done Cupid Jane Dance With Me Never Is Enough It's Over Now Old Apartment, The Only You One Week Peaches & Cream Shoe Box Peaches And Cream Straw Hat U Already Know What A Good Boy Song List Generator® Printed 11/21/2017 Page 1 of 486 Licensed to Greg Reil Reil Entertainment Songs by Artist Karaoke by Artist Title Title 1Barenaked Ladies 20 Fingers When I Fall Short Dick Man 1Beatles, The 2AM Club Come Together Not Your Boyfriend Day Tripper 2Pac Good Day Sunshine California Love (Original Version) Help! 3 Degrees I Saw Her Standing There When Will I See You Again Love Me Do Woman In Love Nowhere Man 3 Dog Night P.S. -

1. Summer Rain by Carl Thomas 2. Kiss Kiss by Chris Brown Feat T Pain 3
1. Summer Rain By Carl Thomas 2. Kiss Kiss By Chris Brown feat T Pain 3. You Know What's Up By Donell Jones 4. I Believe By Fantasia By Rhythm and Blues 5. Pyramids (Explicit) By Frank Ocean 6. Under The Sea By The Little Mermaid 7. Do What It Do By Jamie Foxx 8. Slow Jamz By Twista feat. Kanye West And Jamie Foxx 9. Calling All Hearts By DJ Cassidy Feat. Robin Thicke & Jessie J 10. I'd Really Love To See You Tonight By England Dan & John Ford Coley 11. I Wanna Be Loved By Eric Benet 12. Where Does The Love Go By Eric Benet with Yvonne Catterfeld 13. Freek'n You By Jodeci By Rhythm and Blues 14. If You Think You're Lonely Now By K-Ci Hailey Of Jodeci 15. All The Things (Your Man Don't Do) By Joe 16. All Or Nothing By JOE By Rhythm and Blues 17. Do It Like A Dude By Jessie J 18. Make You Sweat By Keith Sweat 19. Forever, For Always, For Love By Luther Vandros 20. The Glow Of Love By Luther Vandross 21. Nobody But You By Mary J. Blige 22. I'm Going Down By Mary J Blige 23. I Like By Montell Jordan Feat. Slick Rick 24. If You Don't Know Me By Now By Patti LaBelle 25. There's A Winner In You By Patti LaBelle 26. When A Woman's Fed Up By R. Kelly 27. I Like By Shanice 28. Hot Sugar - Tamar Braxton - Rhythm and Blues3005 (clean) by Childish Gambino 29. -
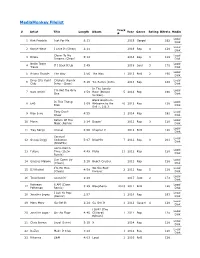
Mediamonkey Filelist
MediaMonkey Filelist Track # Artist Title Length Album Year Genre Rating Bitrate Media # Local 1 Kirk Franklin Just For Me 5:11 2019 Gospel 182 Disk Local 2 Kanye West I Love It (Clean) 2:11 2019 Rap 4 128 Disk Closer To My Local 3 Drake 5:14 2014 Rap 3 128 Dreams (Clean) Disk Nellie Tager Local 4 If I Back It Up 3:49 2018 Soul 3 172 Travis Disk Local 5 Ariana Grande The Way 3:56 The Way 1 2013 RnB 2 190 Disk Drop City Yacht Crickets (Remix Local 6 5:16 T.I. Remix (Intro 2013 Rap 128 Club Intro - Clean) Disk In The Lonely I'm Not the Only Local 7 Sam Smith 3:59 Hour (Deluxe 5 2014 Pop 190 One Disk Version) Block Brochure: In This Thang Local 8 E40 3:09 Welcome to the 16 2012 Rap 128 Breh Disk Soil 1, 2 & 3 They Don't Local 9 Rico Love 4:55 1 2014 Rap 182 Know Disk Return Of The Local 10 Mann 3:34 Buzzin' 2011 Rap 3 128 Macc (Remix) Disk Local 11 Trey Songz Unusal 4:00 Chapter V 2012 RnB 128 Disk Sensual Local 12 Snoop Dogg Seduction 5:07 BlissMix 7 2012 Rap 0 201 Disk (BlissMix) Same Damn Local 13 Future Time (Clean 4:49 Pluto 11 2012 Rap 128 Disk Remix) Sun Come Up Local 14 Glasses Malone 3:20 Beach Cruiser 2011 Rap 128 (Clean) Disk I'm On One We the Best Local 15 DJ Khaled 4:59 2 2011 Rap 5 128 (Clean) Forever Disk Local 16 Tessellated Searchin' 2:29 2017 Jazz 2 173 Disk Rahsaan 6 AM (Clean Local 17 3:29 Bleuphoria 2813 2011 RnB 128 Patterson Remix) Disk I Luh Ya Papi Local 18 Jennifer Lopez 2:57 1 2014 Rap 193 (Remix) Disk Local 19 Mary Mary Go Get It 2:24 Go Get It 1 2012 Gospel 4 128 Disk LOVE? [The Local 20 Jennifer Lopez On the -

Eminem Shady Xv Album Download Eminem Shady Xv Album Download
eminem shady xv album download Eminem shady xv album download. Tracklist: CD1: 01. Eminem – SHADYXV 02. Slaughterhouse & Yelawolf – Psychopath Killer (feat. Eminem) 03. Eminem – Die Alone (feat. Kobe) 04. Bad Meets Evil – Vegas 05. Slaughterhouse – Y’all Ready Know 06. Eminem – Guts Over Fear (feat. Sia) 07. Yelawolf – Down 08. D12 – Bane 09. Eminem – Fine Line 10. Skylar Grey, Eminem & Yelawolf – Twisted 11. Eminem – Right For Me 12. Eminem, Royce da 5’9″, Big Sean, Danny Brown, Dej Loaf & Trick Trick – Detroit Vs. Everybody 13. Yelawolf – Till It’s Gone (Bonus Track) Various - The Shady Bunch album flac. Album · 2013 · 8 Songs. The Dogfight - EP. Roksonix. Let's Do It - Single. PLAY ALL. The Shady Bunch Vol. 2. Released by Shady Nate, Rapbay, Urbanlife Music Jan 2010 23 Tracks. Listen to all songs in high quality & download The Shady Bunch Vol. 2 songs on Gaana. attr("src", $('. de tp. d t img img'). Please disable your ad blocker or, better yet, upgrade to Radio Plus. The music will continue in seconds. Select to cast music to your TV or stereo Please download the Slacker Radio app to complete the upgrade process. GET APP. Please visit ww. lacker. Listen to now in your mobile browser. Listen to The Shady Bunch now. Listen to The Shady Bunch in full in the this site app. Play on this site. You look like someone who appreciates good music. He has never been working harder, Eminem has observed in interviews; in the sense that he's released a 78-minute album and another disc of new music all in the last year, he's right. -

By Song Title
Solar Entertainments Karaoke Song Listing By Song Title 3 Britney Spears 2000s 17 MK 2010s 22 Lily Allen 2000s 39 Queen 1970s 679 Fetty Wap 2010s 711 Beyonce 2010s 1973 James Blunt 2000s 1999 Prince 1980s 2002 Anne Marie 2010s #ThatPower Will.I.Am & Justin Bieber 2010s 007 (Shanty Town) Desmond Dekker & The Aces 1960s 1 800 273 8255 Logic & Alessia Cara & Khalid 2010s 1 Thing Amerie 2000s 10/10 Paolo Nutini 2010s 10000 Hours Dan & Shay & Justin Bieber 2010s 18 & Life Skid Row 1980s 2 Become 1 Spice Girls 1990s 2 Hearts Kylie Minogue 2000s 20th Century Boy T Rex 1970s 21 Guns Green Day 2000s 21st Century Breakdown Green Day 2000s 21st Century Christmas Cliff Richard 2000s 22 (Twenty Two) Taylor Swift 2010s 24K Magic Bruno Mars 2010s 2U David Guetta & Justin Bieber 2010s 3 AM Busted 2000s 3 Nights Dominic Fike 2010s 3 Words Cheryl Cole 2000s 30 Days Saturdays 2010s 34+35 Ariana Grande 2020s 4 44 Jay Z 2010s 4 In The Morning Gwen Stefani 2000s 4 Minutes Madonna & Justin Timberlake 2000s 5 Colours In Her Hair McFly 2000s 5,6,7,8 Steps 1990s 500 Miles (I'm Gonna Be) Proclaimers 1980s 7 Rings Ariana Grande 2010s 7 Things Miley Cyrus 2000s 7 Years Lukas Graham 2010s 74 75 Connells 1990s 9 To 5 Dolly Parton 1980s 90 Days Pink & Wrabel 2010s 99 Red Balloons Nena 1980s A Bad Dream Keane 2000s A Blossom Fell Nat King Cole 1950s A Change Would Do You Good Sheryl Crow 1990s A Cover Is Not The Book Mary Poppins Returns Soundtrack 2010s A Design For Life Manic Street Preachers 1990s A Different Beat Boyzone 1990s A Different Corner George Michael 1980s -

Song Title Artist Genre
Song Title Artist Genre - General The A Team Ed Sheeran Pop A-Punk Vampire Weekend Rock A-Team TV Theme Songs Oldies A-YO Lady Gaga Pop A.D.I./Horror of it All Anthrax Hard Rock & Metal A** Back Home (feat. Neon Hitch) (Clean)Gym Class Heroes Rock Abba Megamix Abba Pop ABC Jackson 5 Oldies ABC (Extended Club Mix) Jackson 5 Pop Abigail King Diamond Hard Rock & Metal Abilene Bobby Bare Slow Country Abilene George Hamilton Iv Oldies About A Girl The Academy Is... Punk Rock About A Girl Nirvana Classic Rock About the Romance Inner Circle Reggae About Us Brooke Hogan & Paul Wall Hip Hop/Rap About You Zoe Girl Christian Above All Michael W. Smith Christian Above the Clouds Amber Techno Above the Clouds Lifescapes Classical Abracadabra Steve Miller Band Classic Rock Abracadabra Sugar Ray Rock Abraham, Martin, And John Dion Oldies Abrazame Luis Miguel Latin Abriendo Puertas Gloria Estefan Latin Absolutely ( Story Of A Girl ) Nine Days Rock AC-DC Hokey Pokey Jim Bruer Clip Academy Flight Song The Transplants Rock Acapulco Nights G.B. Leighton Rock Accident's Will Happen Elvis Costello Classic Rock Accidentally In Love Counting Crows Rock Accidents Will Happen Elvis Costello Classic Rock Accordian Man Waltz Frankie Yankovic Polka Accordian Polka Lawrence Welk Polka According To You Orianthi Rock Ace of spades Motorhead Classic Rock Aces High Iron Maiden Classic Rock Achy Breaky Heart Billy Ray Cyrus Country Acid Bill Hicks Clip Acid trip Rob Zombie Hard Rock & Metal Across The Nation Union Underground Hard Rock & Metal Across The Universe Beatles -

American Music Awards-Chrysler Group LLC, Interscope Records
Contact: Diane Morgan Eileen Wunderlich Chrysler Group LLC and Universal Music's Interscope Records Offer "American Music Awards" Viewers a One-Time Only Television Experience, Featuring Songs From Hit Artists Including Eminem, Fergie, Gwen Stefani and Phillip Phillips Chrysler Group's Artistic and Creative Alignment with Interscope Records Sees Debut of Five Custom Videos, Airing One Night Only during Live Awards Show in One of Company’s Biggest Collaborative Television Partnerships November 24, 2014, Auburn Hills, Mich. - Chrysler Group LLC teamed up with Universal Music Group’s Interscope Records during last night’s American Music Awards (AMAs) to present viewers with a one-time only television experience featuring songs from some of the music industry’s biggest artists. Together, the company and Interscope Records debuted four 30-second and one 60-second customized videos featuring new songs by Eminem (“Guts Over Fear”), Fergie (“L.A.Love (la la)”), Gwen Stefani (“Spark The Fire”) and Phillip Phillips (“Unpack Your Heart”) during the three-hour live broadcast airing on ABC. The videos aired that night only during the AMAs in what is one of Chrysler’s biggest television partnerships. “It’s incredibly rare and special to find a unique partnership where some of the world’s biggest artists and brands align so perfectly, allowing both the opportunity to deliver entertaining and meaningful content to their audiences,” said Olivier Francois, Chief Marketing Officer, Chrysler Group LLC. “Taking part in last night’s American Music Awards was our way of saying ‘thank you’ to this industry and to the many artists, who over the years, have played a meaningful role in helping to develop the unique voice and spirit of each of our brands.” “We’ve had an incredible relationship with Olivier and Chrysler Group over the years,” commented Steve Berman, Interscope Geffen A&M Vice Chairman. -
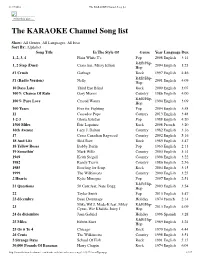
The KARAOKE Channel Song List
11/17/2016 The KARAOKE Channel Song list Print this List ... The KARAOKE Channel Song list Show: All Genres, All Languages, All Eras Sort By: Alphabet Song Title In The Style Of Genre Year Language Dur. 1, 2, 3, 4 Plain White T's Pop 2008 English 3:14 R&B/Hip- 1, 2 Step (Duet) Ciara feat. Missy Elliott 2004 English 3:23 Hop #1 Crush Garbage Rock 1997 English 4:46 R&B/Hip- #1 (Radio Version) Nelly 2001 English 4:09 Hop 10 Days Late Third Eye Blind Rock 2000 English 3:07 100% Chance Of Rain Gary Morris Country 1986 English 4:00 R&B/Hip- 100% Pure Love Crystal Waters 1994 English 3:09 Hop 100 Years Five for Fighting Pop 2004 English 3:58 11 Cassadee Pope Country 2013 English 3:48 1-2-3 Gloria Estefan Pop 1988 English 4:20 1500 Miles Éric Lapointe Rock 2008 French 3:20 16th Avenue Lacy J. Dalton Country 1982 English 3:16 17 Cross Canadian Ragweed Country 2002 English 5:16 18 And Life Skid Row Rock 1989 English 3:47 18 Yellow Roses Bobby Darin Pop 1963 English 2:13 19 Somethin' Mark Wills Country 2003 English 3:14 1969 Keith Stegall Country 1996 English 3:22 1982 Randy Travis Country 1986 English 2:56 1985 Bowling for Soup Rock 2004 English 3:15 1999 The Wilkinsons Country 2000 English 3:25 2 Hearts Kylie Minogue Pop 2007 English 2:51 R&B/Hip- 21 Questions 50 Cent feat. Nate Dogg 2003 English 3:54 Hop 22 Taylor Swift Pop 2013 English 3:47 23 décembre Beau Dommage Holiday 1974 French 2:14 Mike WiLL Made-It feat. -

Eminem Steppin Onto the Scene Tracklist
Eminem steppin onto the scene tracklist click here to download Bassmint Productions – none. Fattest Skinny Kid Alive. If the tape is being sold from the UK, buy with extreme caution because the UK is infamous for selling fake tapes. Find a Bassmint Productions - Steppin' On To The Scene first pressing or reissue. Complete your Bassmint Productions collection. Shop Vinyl and CDs. Bassmint Productions – Steppin' On To The Scene. Genre: Hip Hop. Style: Hardcore Hip-Hop. Year: Tracklist Eminem - Steppin up onto the scene [ VERY RARE] Eminem - Fattest skinny kid alive [VERY RARE] Chaos Kid - Enuff Is Enuff - (Chaos Side) Soul Intent/Bassmint Productions Chaos Kid. Steppin' On To the Scene is the debut EP by local American hip hop group Bassmint Productions, who morphed into Soul Intent in This EP was produced. Soul Intent was an American hip hop group, and consisted of Eminem, Proof, Chaos Kid, Manix and DJ Butter Fingers. They were previously Bassmint Productions between and Contents. 1 Early years: Bassmint Productions; 2 From Soul Intent; 3 Discography. Steppin' On To The Scene () a few homemade/handmade demo tapes, including Steppin' onto the Scene. Tracklist with lyrics of the album STEPPIN' ONTO THE SCENE [] from Soul Intent: Steppin' On To The Scene - Fattest Skinny Kid Alive - Under New. Title, Artist, Rating, Length. 1 · Steppin' On To The Scene · Eminem?:?? 2 · Fattest Skinny Kid Alive · Eminem?:?? 3 · Under New Managment. Style-O-Maniac [Chaos Kid] download; www.doorway.ru Is Enuff [Chaos Kid] (feat. Delirious D) download; www.doorway.ru Have Mercy [Chaos Kid] download; 4. "Steppin' onto the Scene" 2. -

Augsome Karaoke Song List Page 1
AUGSome Karaoke Song List 44 - When Your Heart Stops Beating 112 - Come See Me 112 - Cupid 112 - Dance With Me 112 - It's Over Now 112 - Only You 112 - Peaches And Cream 112 - U Already Know 311 - All Mixed Up 311 - Amber 311 - Beyond The Gray Sky 311 - Creatures (For A While) 311 - Don't Tread On Me 311 - Down 311 - First Straw 311 - Hey You 311 - I'll Be Here Awhile 311 - Love Song 311 - You Wouldn't Believe 411 - Dumb 411 - On My Knees 411 - Teardrops 702 - Get It Together 702 - I Still Love You 702 - Steelo 702 - Where My Girls At 911 - All I Want Is You 911 - How Do You Want Me To Love You 911 - Little Bit More, A 911 - More Than A Woman 911 - Party People (Friday Night) 911 - Private Number 1927 - That's When I Think Of You 1975 - Chocolate 1975 - City 1975 - Love Me 1975 - Robbers 1975 - Sex 1975 - Sound 1975 - Ugh 1 Giant Leap And Jazz Maxi - My Culture 10 Years - Beautiful 10 Years - Through The Iris 10 Years - Wasteland 10,000 Maniacs - Because The Night 10,000 Maniacs - Candy Everybody Wants 10,000 Maniacs - Like The Weather 10,000 Maniacs - More Than This 10,000 Maniacs - These Are The Days 10,000 Maniacs - Trouble Me 100 Proof Aged In Soul - Somebody's Been Sleeping Page 1 AUGSome Karaoke Song List 101 Dalmations - Cruella de Vil 10Cc - Donna 10Cc - Dreadlock Holiday 10Cc - I'm Mandy 10Cc - I'm Not In Love 10Cc - Rubber Bullets 10Cc - Things We Do For Love, The 10Cc - Wall Street Shuffle 112 And Ludacris - Hot And Wet 12 Gauge - Dunkie Butt 12 Stones - Crash 12 Stones - We Are One 1910 Fruitgum Co. -

Shady Records Feirer 15. Års Jubileum Med Album Nr. 15!
26-08-2014 13:44 CEST Shady Records feirer 15. års jubileum med album nr. 15! Eminem og Shady Records feirer labelets 15. års jubileum med deres15. release, "ShadyXV". Dobbeltalbumet slippes 24. november og vil inneholde de største hitsene fra labelet som 50 Cents “In Da Club” og Eminems “Lose Yourself”, samt et album med nytt materiale fra Eminem, Slaughterhouse, Bad Meets Evil, D-12 og Yelawolf, samt noen gjesteartister. Første singel fra albumet "Guts Over Fear" ft Sia er produsert av Emile Haynie som ble tildelt Grammy'n Best Rap Album for sitt samarbeid med Eminem på "Recovery". Låten ble sluppet på iTunes i går og gikk rett til #1: https://itunes.apple.com/no/album/guts-over-fear-feat.-sia- single/id910864306?l=nb I dag er den ute til streaming: http://open.spotify.com/track/0VZs2OQq4axr8GFRdC9nyD & http://wimp.no/album/33650083 Hør låten her og se full pressemelding nedenfor. Se video på YouTube her SHADY RECORDS SET TO RELEASE SHADYXV ON NOVEMBER 28: TWO-CD COLLECTION TO FEATURE THE LABEL’S GREATEST HITS PLUS ALL NEW MATERIAL NEW EMINEM SINGLE OUT TODAY; “GUTS OVER FEAR” LEADS 15th ANNIVERSARY COMPILATION AND IS FEATURED IN UPCOMING FILM, “THE EQUALIZER” SANTA MONICA, CA – August 25, 2014 – Eminem and Shady Records will celebrate the label’s 15th anniversary with its fifteenth release: ShadyXV, a two-CD compilation featuring an album of the label’s greatest hits, including classics such as 50 Cent’s “In Da Club” and Eminem’s “Lose Yourself” plus an album of new material from Eminem, Slaughterhouse, Bad Meets Evil, D-12 and Yelawolf as well as special guest-artist collaborations.