The Application of Mouse and Human Embryonic Stem Cells with Transcriptomics in Alternative Developmental Toxicity Tests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Migration-Selection Balance Drives Genetic Differentiation in Genes Associated with High-Altitude Function in the Speckled
GBE Migration-Selection Balance Drives Genetic Differentiation in Genes Associated with High-Altitude Function in the Speckled Teal (Anas flavirostris) in the Andes Downloaded from https://academic.oup.com/gbe/article-abstract/10/1/14/4685994 by University of Texas at El Paso user on 18 January 2019 Allie M. Graham1,*, Philip Lavretsky2, Violeta Munoz-Fuentes~ 3,4,AndyJ.Green4,RobertE.Wilson5,and Kevin G. McCracken1,5,6,7 1Department of Biology, University of Miami 2Department of Biological Sciences, University of Texas El Paso 3European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, United Kingdom 4Estacion Biologica de Donana,~ EBD-CSIC, Sevilla, Spain 5Institute of Arctic Biology and University of Alaska Museum, University of Alaska, Fairbanks 6Rosenstiel School of Marine and Atmospheric Sciences, University of Miami 7John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine *Corresponding author: E-mail: [email protected]. Accepted: November 30, 2017 Data deposition: DNA sequence GenBank accession information is available in supplementary table S2, Supplementary Material online. Parsed Illumina reads for the RAD-Seq data sets are already available through NCBI short read archive (SRA PRJEB11624). Abstract Local adaptation frequently occurs across populations as a result of migration-selection balance between divergent selective pressures and gene flow associated with life in heterogeneous landscapes. Studying the effects of selection and gene flow on the adaptation process can be achieved in systems that have recently colonized extreme environments. This study utilizes an endemic South American duck species, the speckled teal (Anas flavirostris), which has both high- and low-altitude populations. -

A Hierarchical Machine Learning Model to Discover Gleason Grade-Specific Biomarkers in Prostate Cancer
Henry Ford Health System Henry Ford Health System Scholarly Commons Urology Articles Urology 12-11-2019 A Hierarchical Machine Learning Model to Discover Gleason Grade-Specific Biomarkers in Prostate Cancer. Osama Hamzeh Abedalrhman Alkhateeb Zhuoran Zheng Srinath Kandalam Crystal Leung See next page for additional authors Follow this and additional works at: https://scholarlycommons.henryford.com/urology_articles Recommended Citation Hamzeh O, Alkhateeb A, Zheng JZ, Kandalam S, Leung C, Atikukke G, Cavallo-Medved D, Palanisamy N, and Rueda L. A Hierarchical Machine Learning Model to Discover Gleason Grade-Specific Biomarkers in Prostate Cancer. Diagnostics (Basel) 2019; 9(4). This Article is brought to you for free and open access by the Urology at Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Urology Articles by an authorized administrator of Henry Ford Health System Scholarly Commons. Authors Osama Hamzeh, Abedalrhman Alkhateeb, Zhuoran Zheng, Srinath Kandalam, Crystal Leung, Govindaraja Atikukke, Dora Cavallo-Medved, Nallasivam Palanisamy, and Luis Rueda This article is available at Henry Ford Health System Scholarly Commons: https://scholarlycommons.henryford.com/ urology_articles/341 diagnostics Article A Hierarchical Machine Learning Model to Discover Gleason Grade-Specific Biomarkers in Prostate Cancer Osama Hamzeh 1,†, Abedalrhman Alkhateeb 1,*,† , Julia Zhuoran Zheng 1, Srinath Kandalam 2, Crystal Leung 3, Govindaraja Atikukke 4, Dora Cavallo-Medved 2, Nallasivam Palanisamy 5,* and Luis Rueda -

Human FGFBP1 ELISA Kit (ARG82056)
Product datasheet [email protected] ARG82056 Package: 96 wells Human FGFBP1 ELISA Kit Store at: 4°C Summary Product Description Human FGFBP1 ELISA Kit is an Enzyme Immunoassay kit for the quantification of Human FGFBP1 in serum, plasma and cell culture supernatants. Tested Reactivity Hu Tested Application ELISA Target Name FGFBP1 Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm. Sensitivity 11.7 pg/ml Sample Type Serum, plasma and cell culture supernatants. Standard Range 23.4 - 1500 pg/ml Sample Volume 100 µl Alternate Names Fibroblast growth factor-binding protein 1; 17 kDa heparin-binding growth factor-binding protein; FGF- BP; FGF-binding protein 1; HBp17; FGFBP; 17 kDa HBGF-binding protein; FGFBP-1; HBP17; FGF-BP1 Application Instructions Assay Time 4.5 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. Keep microplate wells sealed in a dry bag with desiccants. Do not expose test reagents to heat, sun or strong light during storage and usage. Please refer to the product user manual for detail temperatures of the components. Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Gene Symbol FGFBP1 Gene Full Name fibroblast growth factor binding protein 1 Background This gene encodes a secreted fibroblast growth factor carrier protein. The encoded protein plays a critical role in cell proliferation, differentiation and migration by binding to fibroblast growth factors and potentiating their biological effects on target cells. The encoded protein may also play a role in tumor growth as an angiogenic switch molecule, and expression of this gene has been associated with several types of cancer including pancreatic and colorectal adenocarcinoma. -
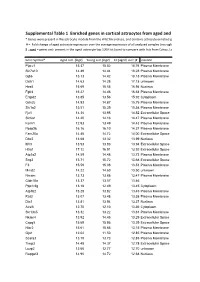
Supplemental Table 1 Enriched Genes in Cortical Astrocytes from Aged
Supplemental Table 1 Enriched genes in cortical astrocytes from aged and young-adult mice * Genes were present in the astrocyte module from the WGCNA analysis, and contains astrocyte enriched genes compared to microglia and oligodendrocytes # = Fold change of aged astrocyte expression over the average expression of all analyzed samples (microglia, astrocytes: young, old, with and without myelin contamination) $ ; aged = genes only present in the aged astrocyte top 1000 list (used to compare with lists from Cahoy, Lovatt, Doyle; see Fig. 4B), all = genes present in all astrocyte top 1000 lists Gene Symbol* Aged astr. (log2) Young astr.(log2) FC (aged/ aver.)# Location Ptprz1 15.37 15.02 18.76 Plasma Membrane Slc7a10 14.49 14.44 18.28 Plasma Membrane Gjb6 15.13 14.42 18.18 Plasma Membrane Dclk1 14.63 14.28 17.18 unknown Hes5 15.69 15.55 16.94 Nucleus Fgfr3 15.27 14.46 16.54 Plasma Membrane Entpd2 13.85 13.56 15.92 Cytoplasm Grin2c 14.93 14.87 15.75 Plasma Membrane Slc1a2 15.51 15.39 15.58 Plasma Membrane Fjx1 14.36 13.98 14.52 Extracellular Space Slc6a1 14.20 14.16 14.47 Plasma Membrane Kcnk1 12.93 13.49 14.43 Plasma Membrane Ppap2b 16.16 16.10 14.37 Plasma Membrane Fam20a 14.48 14.72 14.00 Extracellular Space Dbx2 13.68 13.32 13.99 Nucleus Itih3 13.93 13.93 13.94 Extracellular Space Htra1 17.12 16.91 13.92 Extracellular Space Atp1a2 14.59 14.48 13.73 Plasma Membrane Scg3 15.71 15.72 13.68 Extracellular Space F3 15.59 15.08 13.51 Plasma Membrane Mmd2 14.22 14.60 13.50 unknown Nrcam 13.73 13.88 13.47 Plasma Membrane Cldn10a 13.37 13.57 13.46 -

Nuclear Organization and the Epigenetic Landscape of the Mus Musculus X-Chromosome Alicia Liu University of Connecticut - Storrs, [email protected]
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 8-9-2019 Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome Alicia Liu University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Liu, Alicia, "Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome" (2019). Doctoral Dissertations. 2273. https://opencommons.uconn.edu/dissertations/2273 Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome Alicia J. Liu, Ph.D. University of Connecticut, 2019 ABSTRACT X-linked imprinted genes have been hypothesized to contribute parent-of-origin influences on social cognition. A cluster of imprinted genes Xlr3b, Xlr4b, and Xlr4c, implicated in cognitive defects, are maternally expressed and paternally silent in the murine brain. These genes defy classic mechanisms of autosomal imprinting, suggesting a novel method of imprinted gene regulation. Using Xlr3b and Xlr4c as bait, this study uses 4C-Seq on neonatal whole brain of a 39,XO mouse model, to provide the first in-depth analysis of chromatin dynamics surrounding an imprinted locus on the X-chromosome. Significant differences in long-range contacts exist be- tween XM and XP monosomic samples. In addition, XM interaction profiles contact a greater number of genes linked to cognitive impairment, abnormality of the nervous system, and abnormality of higher mental function. This is not a pattern that is unique to the imprinted Xlr3/4 locus. Additional Alicia J. Liu - University of Connecticut - 2019 4C-Seq experiments show that other genes on the X-chromosome, implicated in intellectual disability and/or ASD, also produce more maternal contacts to other X-linked genes linked to cognitive impairment. -

High-Density P300 Enhancers Control Cell State Transitions Steven Witte1,2, Allan Bradley2, Anton J
Witte et al. BMC Genomics (2015) 16:903 DOI 10.1186/s12864-015-1905-6 RESEARCH ARTICLE Open Access High-density P300 enhancers control cell state transitions Steven Witte1,2, Allan Bradley2, Anton J. Enright3* and Stefan A. Muljo1* Abstract Background: Transcriptional enhancers are frequently bound by a set of transcription factors that collaborate to activate lineage-specific gene expression. Recently, it was appreciated that a subset of enhancers comprise extended clusters dubbed stretch- or super-enhancers (SEs). These SEs are located near key cell identity genes, and enriched for non-coding genetic variations associated with disease. Previously, SEs have been defined as having the highest density of Med1, Brd4 or H3K27ac by ChIP-seq. The histone acetyltransferase P300 has been used as a marker of enhancers, but little is known about its binding to SEs. Results: We establish that P300 marks a similar SE repertoire in embryonic stem cells as previously reported using Med1 and H3K27ac. We also exemplify a role for SEs in mouse T helper cell fate decision. Similarly, upon activation of macrophages by bacterial endotoxin, we found that many SE-associated genes encode inflammatory proteins that are strongly up-regulated. These SEs arise from small, low-density enhancers in unstimulated macrophages. We also identified expression quantitative trait loci (eQTL) in human monocytes that lie within such SEs. In macrophages and Th17 cells, inflammatory SEs can be perturbed either genetically or pharmacologically thus revealing new avenues to target inflammation. Conclusions: Our findings support the notion that P300-marked SEs can help identify key nodes of transcriptional control during cell fate decisions. -

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Comparison of the Agilent, ROMA/Nimblegen and Illumina
BMC Genomics BioMed Central Research article Open Access Comparison of the Agilent, ROMA/NimbleGen and Illumina platforms for classification of copy number alterations in human breast tumors LO Baumbusch*1,2,3, J Aarøe1,4, FE Johansen1, J Hicks5, H Sun6, L Bruhn6, K Gunderson7, B Naume8, VN Kristensen1,4, K Liestøl3,9, A-L Børresen-Dale1,4 and OC Lingjærde3,9 Address: 1Department of Genetics, Institute for Cancer Research, Norwegian Radium Hospital, Rikshospitalet University Hospital, 0310 Oslo, Norway, 2Department of Pathology, Norwegian Radium Hospital, Rikshospitalet University Hospital, 0310 Oslo, Norway, 3Biomedical Research Group, Department of Informatics, University of Oslo, P.O. Box 1080 Blindern, 0316 Oslo, Norway, 4Faculty Division The Norwegian Radium Hospital, University of Oslo, Oslo, Norway, 5Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA, 6Agilent Technologies, Inc., 5301 Stevens Creek Blvd, Santa Clara, CA 95052, USA, 7Illumina, Inc., 9885 Towne Centre Drive, San Diego, CA 92121, USA, 8Department of Oncology, Norwegian Radium Hospital, Rikshospitalet University Hospital, 0310 Oslo, Norway and 9Centre for Cancer Biomedicine, University of Oslo, Oslo, Norway Email: LO Baumbusch* - [email protected]; J Aarøe - [email protected]; FE Johansen - Fredrik.Ekeberg.Johansen@rr- research.no; J Hicks - [email protected]; H Sun - [email protected]; L Bruhn - [email protected]; K Gunderson - [email protected]; B Naume - [email protected]; VN Kristensen - [email protected]; K Liestøl - [email protected]; A-L Børresen-Dale - [email protected]; OC Lingjærde - [email protected] * Corresponding author Published: 8 August 2008 Received: 3 December 2007 Accepted: 8 August 2008 BMC Genomics 2008, 9:379 doi:10.1186/1471-2164-9-379 This article is available from: http://www.biomedcentral.com/1471-2164/9/379 © 2008 Baumbusch et al; licensee BioMed Central Ltd. -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

Discovery of Resistance Pathways to Fibroblast Growth Factor Receptor Inhibition in Bladder Cancer
bioRxiv preprint doi: https://doi.org/10.1101/183293; this version posted August 31, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Discovery of Resistance Pathways to Fibroblast Growth Factor Receptor inhibition in Bladder Cancer Sumanta K Pal, Miaoling He, Jeremy O Jones Department of Medical Oncology, City of Hope National Medical Center, Duarte, CA Running Title: FGFRi resistance in bladder cancer Corresponding Author and to whom reprint requests should be made: Jeremy Jones 1500 E Duarte Rd Beckman 2310 Duarte, CA 91010 Phone: 626-256-4673 ext 80270 Fax: 626-471-3902 Email: [email protected] Figures: 4 Total Words (Abstract Words): 3438 (218) bioRxiv preprint doi: https://doi.org/10.1101/183293; this version posted August 31, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract: Background: Aberrant fibroblast growth factor receptor (FGFR) signaling drives the growth of many bladder cancers. NVP-BGJ398 is a small molecule with potent inhibitory activity of FGFRs 1, 2, and 3, and has been shown to selectively inhibit the growth of bladder cancer cell lines that over-express FGFR3 or have oncogenic FGFR3 fusions. As with many agents targeting receptor tyrosine kinases, resistance is known to develop. Objective: We sought to identify potential mechanisms of resistance to NVP-BGJ398 in cell culture models of bladder cancer. Methods: RT-112 bladder cancer cell lines were derived that were resistant to growth in 3uM NVP-BGJ398. -

Anti-FGFBP1 Antibody (ARG41185)
Product datasheet [email protected] ARG41185 Package: 100 μl anti-FGFBP1 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes FGFBP1 Tested Reactivity Hu, Ms, Rat Tested Application WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name FGFBP1 Antigen Species Human Immunogen Recombinant fusion protein corresponding to aa. 24-234 of Human FGFBP1 (NP_005121.1). Conjugation Un-conjugated Alternate Names Fibroblast growth factor-binding protein 1; 17 kDa heparin-binding growth factor-binding protein; FGF- BP; FGF-binding protein 1; HBp17; FGFBP; 17 kDa HBGF-binding protein; FGFBP-1; HBP17; FGF-BP1 Application Instructions Application table Application Dilution WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control A549 Calculated Mw 26 kDa Observed Size 30 kDa Properties Form Liquid Purification Affinity purified. Buffer PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Gene Symbol FGFBP1 Gene Full Name fibroblast growth factor binding protein 1 Background This gene encodes a secreted fibroblast growth factor carrier protein.