Supplement of OH and HO2 Radical Chemistry in a Midlatitude Forest: Measurements and Model Comparisons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Borneol - Camphor - Isoborneol
EXP. 35 A & B OXIDATION-REDUCTION SCHEME: BORNEOL - CAMPHOR - ISOBORNEOL LEARNING OBJECTIVES: To illustrate the concepts of oxidation and reduction in organic chemistry, to illustrate the stereochemical effects of these reactions in certain systems, to use IR spectroscopy to characterize diastereomers and monitor reactions. QUIZ PREPARATION: Recitation notes and readings assigned in the syllabus. OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY The concepts of oxidation and reduction in chemistry are fundamentally related to the loss and gain of electrons, respectively. For a specific atom, oxidation brings about an increase in the oxidation number, whereas reduction does the opposite. This is of course true in organic reactions as well, but it is frequently less clear where the gain or loss of electrons is taking place. For this reason, the concepts of oxidation and reduction are addressed from a different perspective in organic chemistry. Since carbon is the most important element in organic chemistry, we can define oxidation as a process whereby carbon gains bonds to more electronegative atoms. These can be any atoms more electronegative than carbon, such as chlorine or sulphur, but the most commonly seen in organic oxidations is oxygen. The more bonds to oxygen, the higher the oxidation state of the carbon involved. Examples of oxidation reactions are those that convert alkanes into alcohols, or alcohols into carbonyl compounds. The functional groups that contain the most highly oxidized form of carbon in organic compounds are the carboxylic acids and their derivatives. Reduction is the opposite of oxidation. We can define reduction as a process whereby carbon adds bonds to less electronegative atoms. -

Butenes Separation, Supp. A
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California 94025 Abstract Process Economics Program Report No. 71A BUTTLENES (October 1982) Demand is fast increasing for lsobutylene, especially that used in manufacturing methyl tertiary-butyl ether, and for high purity butene-1 to use as a copolymer in linear low density polyethylene. Because of their wide availability, mixed butane-butylene streams from oleflns plants and petroleum refineries are being increasingly fed to plants to separate butylenes for use in chemicals. This first supplement to Report No. 71 updates demand projections, production capacities, and separation techniques for high purity butene-1 and lsobutylene. The processes that are now available for separating and purifying both butene-1 and lsobutylene from mixed butyl- ene streams are evaluated and compared. Other procedures for obtaining butylenes, such as dehydrogenatlon, lsomerleatlon, and disproportion&ion, are not updated in this report. PEP’81 JLC Report No. 71A - BUTYLENES SUPPLEMENT A by JOHN L. CHADWICK I I October 1982 f-F0 0 A private report by the m PROCESS ECONOMICS PROGRAM 0 Menlo Park, California 94025 0 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing in marketing research. The CHEMICALECONOMICS HANDBOOK Program covers most major chemicals and chemical products produced in the United States and the WORLDPETROCHEMICALS Program covers major hkdrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTORYOF CHEMICALPRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . -

SAFETY DATA SHEET Cyclopentane
SAFETY DATA SHEET Cyclopentane Section 1. Identification GHS product identifier : Cyclopentane Chemical name : cyclopentane Other means of : cyclopentane (dot); pentamethylene identification Product use : Synthetic/Analytical chemistry. Synonym : cyclopentane (dot); pentamethylene SDS # : 001124 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture AQUATIC HAZARD (ACUTE) - Category 3 AQUATIC HAZARD (LONG-TERM) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Highly flammable liquid and vapor. May form explosive mixtures in Air. Harmful to aquatic life with long lasting effects. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Avoid release to the environment. Response : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower. Storage : Store in a well-ventilated place. Keep cool. Disposal : Dispose of contents and container in accordance with all local, regional, national and international regulations. Hazards not otherwise : None known. -
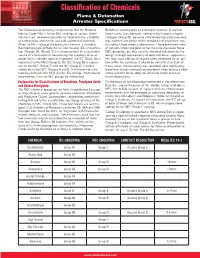
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

A New Source of Natural D-Borneol and Its Characteristic
Journal of Medicinal Plants Research Vol. 5(15), pp. 3440-3447, 4 August, 2011 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2011 Academic Journals Full Length Research Paper A new source of natural D-borneol and its characteristic Lei Chen 1, Jianyu Su 1*, Lin Li 1, Bing Li 1 and Wang Li 2 1Department of Food Science and Technology, South China University of Technology, 510642 Guangzhou, Peoples Republic of China. 2Food Safety Key Laboratory of Guangdong Province, College of Food Science, South China Agricultural University, 510642 Guangzhou, Peoples Republic of China. Accepted 28 February, 2011 The essential oils from leaves of Mei Pian tree (a Cinnamomun burmannii physiological type), were obtained by hydro-distillation and analyzed by gas chromatography and mass spectrometry (GC/MS). Forty compounds representing 99.61% of the total oil containing D-borneol (78.6%) as the major component were obtained. D-borneol was purified by sublimation, and the characteristic of the refined D-borneol was compared with standard D-borneol and synthetic borneol by infrared spectra analysis and optical activity analysis. The chemical composition and the optical rotation of refined D-borneol from leaves of Mei Pian tree were extremely similar to the standard D-borneol and materially surpass synthetic borneol. Antioxidant activities of the refined D-borneol and standard D-borneol were determined by testing their DPPH and hydroxyl radicals scavenging activities and the reducing power. The refined D-borneol and standard D-borneol had the same antioxidant activity. They exhibited higher hydroxyl radicals scavenging activity, but weaker reducing power, compared with the synthetic borneol. -

MSDS Cyclopentane (1).Pdf
MATERIAL SAFETY DATA SHEET (MSDS) CYCLOPENTANE 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION SUBSTANCE OR PREPATION TRADE NAME Cyclopentane CHEMICAL CLASSIFICATION Cycloparaffin, Naphthene COMPANY/ UNDERTAKING NAME AND Haldia Petrochemicals Limited, ADDRESS PO Box No 12, Haldia Plant PO Durgachak, Dist Midnapore West Bengal, India PIN 721 602 TELEPHONE 091-3224-274384 / 274400 EMERGENCY TELEPHONE NUMBER 091-3224-275916 2. COMPOSTION AND INFORMATION ON INGREDIENTS CHEMICAL CHEMICLAL CONTENT CAS EXPOSURE LIMITS IN AIR NAME FORMULA NUMBER (ppm) ACGIH ACGIH IDLH TLV- TLV- TWA STEL Cyclopentane C5H10 98.20 wt% 287-92-3 600 NA NA 2,2 Dimethyl (CH3)3CCH2CH3 0.28 wt% 75-83-2 500 1000 NA butane n- pentane n- C5H12 1.47 wt% 109-66-0 600 750 NA i- Pentane i- C5H12 0.05 wt% 78-78-4 600 750 NA Total Sulpher S < 1.0 wt. ppm 7704-34-9 NA NA NA 3. HAZARD CLASSIFICATION Highly flammable liquid and vapour. Vapour EMERGENCY OVERVIEW may cause flash fire. Harmful or Fatal is enter lungs and cause damage POTENTIAL HEALTH HAZARDS EYE SKIN INHALATION INGESTION OTHERS ACUTE To cause Not expected Dizziness, Abdominal prolonged or to be harmful headache, pain, significant to internal nausea, diarrhoea, eye irritation organs if Unconsciousness, dizziness, absorbed weakness nausea, sore through the throat skin. CHRONIC Repeated or prolonged contact with skin may cause dermatitis NFPA HAZARD HEALTH FLAMMABILITY REACTIVITY SPECIAL SIGNALS 2 3 0 - HAZCHEM CODE 3YE GHS-Classification Flammable liquids Category 2 Aspiration hazard Category 1 Chronic aquatic toxicity, Category 3 Acute aquatic toxicity, Category 3 Target Organ Systemic Toxicant - Single exposure, Category 3, Inhalation, Central nervous system Document Compiled By Approved By Issue No Rev. -

Natural Borneol Recycling from Cinnamomum Camphor Chvar
Chen et al Tropical Journal of Pharmaceutical Research September 2014; 13 (9): 1463-1470 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved . Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v13i9.12 Original Research Article Natural Borneol Recycling from Cinnamomum camphor chvar. Borneol Oil Residue by Fractional Distillation and Recrystallization Xiao Ying Chen 1, Xiao Ning Zhao 2, Hui Fang Zeng 2, Jian Hui Xie 1,3 , Xiao Lu Chen 2, Yong Zhuo Liang 1, Qi Duan Wu 2, Zi Ren Su 1,5 , Hong Feng Wang 4* and Xiao Ping Lai 1,5 * 1School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou 510006, 2The First Affiliated Hospital of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, 3The Second Affiliated Hospital of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, 4Biotechnology Division, Guangdong Academy of Forestry, Guangzhou, 5Dongguan Mathematical Engineering Academy of Chinese Medicine, Guangzhou University of Chinese Medicine, Dongguan, 523808, China *For correspondence: Email: [email protected], [email protected]; Tel: +86-20-39358517; Fax: +86-20-39358390 Received: 10 May 2014 Revised accepted: 7 August 2014 Abstract Purpose: To establish an efficient method to recycle natural borneol from Cinnamomum camphor chvar. Borneol oil residue. Methods: The fractions and raffinate of the oil residue of Cinnamomum camphor chvar. Borneol were obtained by fractional distillation, and analyzed by gas chromatography with flame ionization detector (GC-FID) and gas chromatography- mass spectrometry (GC-MS). Natural borneol was purified by recrystallization and suction filtration. -

Supplement of Compilation and Evaluation of Gas Phase Diffusion Coefficients of Reactive Trace Gases in the Atmosphere
Supplement of Atmos. Chem. Phys., 15, 5585–5598, 2015 http://www.atmos-chem-phys.net/15/5585/2015/ doi:10.5194/acp-15-5585-2015-supplement © Author(s) 2015. CC Attribution 3.0 License. Supplement of Compilation and evaluation of gas phase diffusion coefficients of reactive trace gases in the atmosphere: Volume 2. Diffusivities of organic compounds, pressure-normalised mean free paths, and average Knudsen numbers for gas uptake calculations M. J. Tang et al. Correspondence to: M. J. Tang ([email protected]) and M. Kalberer ([email protected]) The copyright of individual parts of the supplement might differ from the CC-BY 3.0 licence. Table of Contents 1 Alkanes and cycloalkanes ............................................................................................ 1 1.1 CH 4 (methane), C 2H6 (ethane), and C 3H8 (propane) ............................................. 1 1.2 C 4H10 (butane, methyl propane) ............................................................................ 3 1.3 C 5H12 (n-pentane, methyl butane, dimethyl butane) ............................................. 5 1.4 C 6H14 (n-hexane, 2,3-dimethyl butane) ................................................................ 7 1.5 C 7H16 (n-heptane, 2,4-dimethyl pentane).............................................................. 9 1.6 C 8H18 (n-octane, 2,2,4-trimethyl pentane) .......................................................... 11 1.7 C 9H20 (n-nonane), C 10 H22 (n-decane, 2,3,3-trimethyl heptane) and C 12 H26 (n- dodecane) ................................................................................................................. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Cyclopentane Cyp
CYCLOPENTANE CYP CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: Commercial; 60% (remainder Common Synonyms Watery liquid Colorless Mild, sweet odor < 20°F C.C. consists of hydrocarbons of similar boiling Pentamethylene 4.2 Flammable Limits in Air: (approx.) 1.1%- point); Research: 99+% 8.7% 7.2 Storage Temperature: Ambient Floats on water. Flammable, irritating vapor is produced. 4.3 Fire Extinguishing Agents: Dry 7.3 Inert Atmosphere: No requirement chemical, foam, carbon dioxide Keep people away. 7.4 Venting: Pressure-vacuum Avoid inhalation. 4.4 Fire Extinguishing Agents Not to Be Shut off ingition sources. Call fire department. Used: Water may be ineffective. 7.5 IMO Pollution Category: (C) Evacuate area in case of large discharge. 4.5 Special Hazards of Combustion 7.6 Ship Type: 3 Notify local health and pollution control agencies. Products: Not pertinent 7.7 Barge Hull Type: Currently not available Protect water intakes. 4.6 Behavior in Fire: Containers may explode. FLAMMABLE. 8. HAZARD CLASSIFICATIONS Fire 4.7 Auto Ignition Temperature: 682°F Containers may explode in fire. 8.1 49 CFR Category: Flammable liquid Flashback along vapor trail may occur. 4.8 Electrical Hazards: Not pertinent 8.2 49 CFR Class: 3 Vapor may explode if ignited in an enclosed area. 4.9 Burning Rate: 7.9 mm/min. 8.3 49 CFR Package Group: II Extinguish with dry chemicals, foam or carbon dioxide. 4.10 Adiabatic Flame Temperature: Currently Water may be ineffective on fire. not available 8.4 Marine Pollutant: No Cool exposed containers with water. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Flammable Liquefied Gas Mixture: 2-Methylpentane / 2-Methylhexane / 2, 3-Dimethylheptane / Benzene / Cyclohexane / Cyclopentane / Dodecane / Ethyl Benzene / Ethyl Cyclohexane / Hexane / Isobutane / Isobutyl Cyclohexane / Isooctane / Isopentane / Isopropylcyclohexane / Methyl Cyclohexane / N-Butane / N-Butyl Benzene / N-Decane / N-Heptane / N-Nonane / N-Octane / N-Pentane / N-Propyl Benzene / Toluene Section 1. Identification GHS product identifier : Flammable Liquefied Gas Mixture: 2-Methylpentane / 2-Methylhexane / 2, 3-Dimethylheptane / Benzene / Cyclohexane / Cyclopentane / Dodecane / Ethyl Benzene / Ethyl Cyclohexane / Hexane / Isobutane / Isobutyl Cyclohexane / Isooctane / Isopentane / Isopropylcyclohexane / Methyl Cyclohexane / N-Butane / N-Butyl Benzene / N-Decane / N-Heptane / N-Nonane / N-Octane / N-Pentane / N-Propyl Benzene / Toluene Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 021986 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Liquefied gas SKIN IRRITATION - Category 2 GERM CELL MUTAGENICITY - Category 1 CARCINOGENICITY - Category 1 TOXIC TO REPRODUCTION (Fertility) - Category 2 TOXIC TO REPRODUCTION (Unborn child) - Category 2 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) - Category 1 AQUATIC HAZARD (ACUTE) - Category 2 AQUATIC HAZARD (LONG-TERM) - Category 2 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. May form explosive mixtures with air.