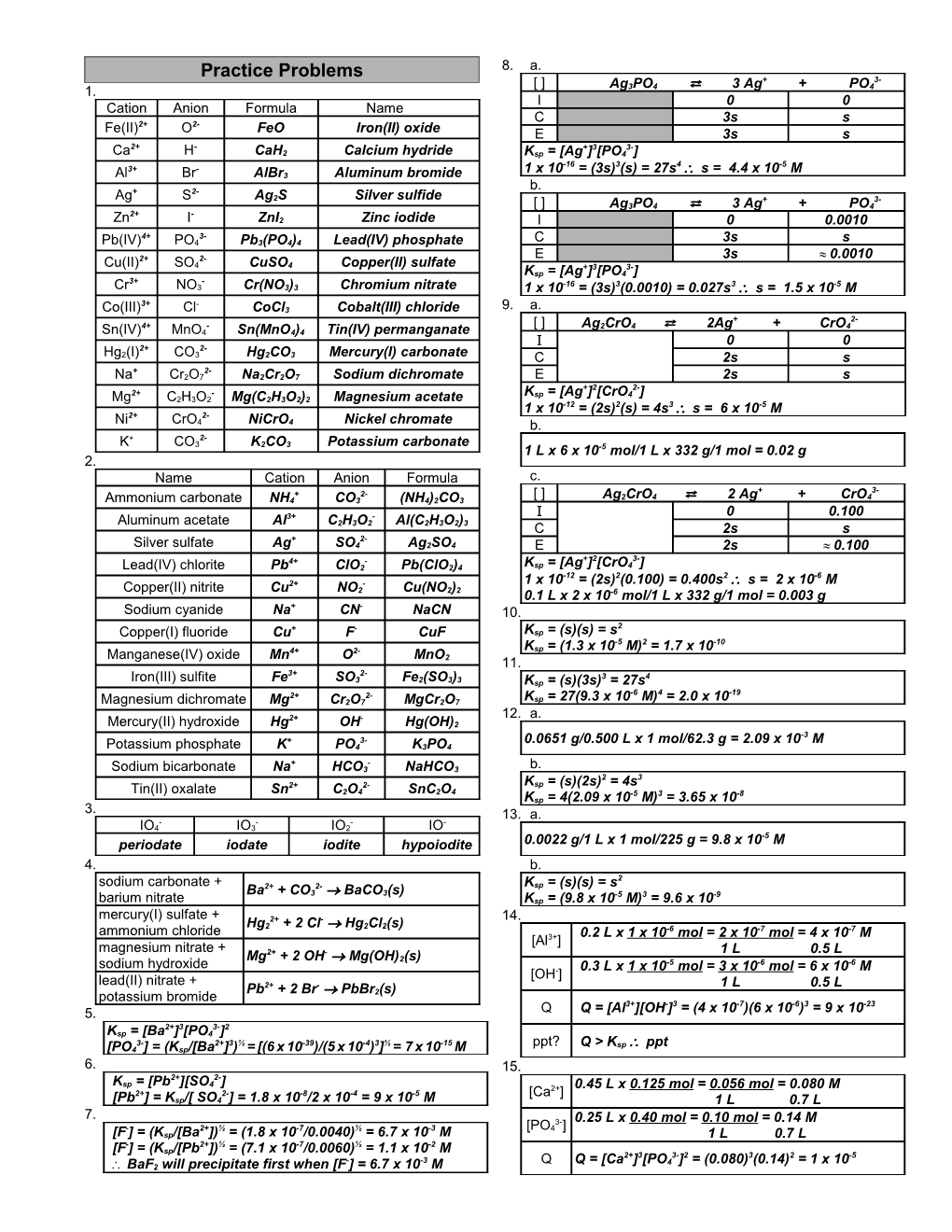
8. a. Practice Problems + 3- [ ] Ag3PO4 3 Ag + PO4 1. ⇄ I 0 0 Cation Anion Formula Name 2+ 2- C 3s s Fe(II) O FeO Iron(II) oxide E 3s s 2+ - + 3 3- Ca H CaH2 Calcium hydride Ksp = [Ag ] [PO4 ] -16 3 4 -5 3+ - 1 x 10 = (3s) (s) = 27s s = 4.4 x 10 M Al Br AlBr3 Aluminum bromide + 2- b. Ag S Ag2S Silver sulfide + 3- [ ] Ag3PO4 ⇄ 3 Ag + PO4 2+ - Zn I ZnI2 Zinc iodide I 0 0.0010 4+ 3- Pb(IV) PO4 Pb3(PO4)4 Lead(IV) phosphate C 3s s 2+ 2- E 3s 0.0010 Cu(II) SO4 CuSO4 Copper(II) sulfate + 3 3- Ksp = [Ag ] [PO4 ] 3+ - Cr NO3 Cr(NO3)3 Chromium nitrate 1 x 10-16 = (3s)3(0.0010) = 0.027s3 s = 1.5 x 10-5 M 3+ - Co(III) Cl CoCl3 Cobalt(III) chloride 9. a. + 2- 4+ - [ ] Ag2CrO4 2Ag + CrO4 Sn(IV) MnO4 Sn(MnO4)4 Tin(IV) permanganate ⇄ I 0 0 2+ 2- Hg2(I) CO3 Hg2CO3 Mercury(I) carbonate C 2s s + 2- Na Cr2O7 Na2Cr2O7 Sodium dichromate E 2s s + 2 2- 2+ - Ksp = [Ag ] [CrO4 ] Mg C2H3O2 Mg(C2H3O2)2 Magnesium acetate -12 2 3 -5 2+ 2- 1 x 10 = (2s) (s) = 4s s = 6 x 10 M Ni CrO4 NiCrO4 Nickel chromate b. + 2- K CO3 K2CO3 Potassium carbonate 1 L x 6 x 10-5 mol/1 L x 332 g/1 mol = 0.02 g 2. Name Cation Anion Formula c. + 2- [ ] + 3- Ammonium carbonate NH4 CO3 (NH4)2CO3 Ag2CrO4 ⇄ 2 Ag + CrO4 3+ - I 0 0.100 Aluminum acetate Al C2H3O2 Al(C2H3O2)3 C 2s s + 2- Silver sulfate Ag SO4 Ag2SO4 E 2s 0.100 4+ - + 2 3- Lead(IV) chlorite Pb ClO2 Pb(ClO2)4 Ksp = [Ag ] [CrO4 ] -12 2 2 -6 2+ - 1 x 10 = (2s) (0.100) = 0.400s s = 2 x 10 M Copper(II) nitrite Cu NO2 Cu(NO2)2 0.1 L x 2 x 10-6 mol/1 L x 332 g/1 mol = 0.003 g Sodium cyanide Na+ CN- NaCN 10. + - 2 Copper(I) fluoride Cu F CuF Ksp = (s)(s) = s -5 2 -10 4+ 2- Ksp = (1.3 x 10 M) = 1.7 x 10 Manganese(IV) oxide Mn O MnO2 11. 3+ 2- 3 4 Iron(III) sulfite Fe SO3 Fe2(SO3)3 Ksp = (s)(3s) = 27s 2+ 2- -6 4 -19 Magnesium dichromate Mg Cr2O7 MgCr2O7 Ksp = 27(9.3 x 10 M) = 2.0 x 10 2+ - 12. a. Mercury(II) hydroxide Hg OH Hg(OH)2 -3 + 3- 0.0651 g/0.500 L x 1 mol/62.3 g = 2.09 x 10 M Potassium phosphate K PO4 K3PO4 + - Sodium bicarbonate Na HCO3 NaHCO3 b. 2 3 2+ 2- Ksp = (s)(2s) = 4s Tin(II) oxalate Sn C2O4 SnC2O4 -5 3 -8 Ksp = 4(2.09 x 10 M) = 3.65 x 10 3. 13. a. - - - - IO4 IO3 IO2 IO -5 periodate iodate iodite hypoiodite 0.0022 g/1 L x 1 mol/225 g = 9.8 x 10 M 4. b. 2 sodium carbonate + 2+ 2- Ksp = (s)(s) = s Ba + CO3 BaCO3(s) -5 3 -9 barium nitrate Ksp = (9.8 x 10 M) = 9.6 x 10
mercury(I) sulfate + 2+ - 14. Hg2 + 2 Cl Hg2Cl2(s) ammonium chloride 0.2 L x 1 x 10 -6 mol = 2 x 10 -7 mol = 4 x 10-7 M [Al3+] magnesium nitrate + 2+ - 1 L 0.5 L Mg + 2 OH Mg(OH)2(s) sodium hydroxide 0.3 L x 1 x 10 -5 mol = 3 x 10 -6 mol = 6 x 10-6 M [OH-] lead(II) nitrate + 2+ - 1 L 0.5 L Pb + 2 Br PbBr2(s) potassium bromide 3+ - 3 -7 -6 3 -23 5. Q Q = [Al ][OH ] = (4 x 10 )(6 x 10 ) = 9 x 10 2+ 3 3- 2 Ksp = [Ba ] [PO4 ] 3- 2+ 3 ½ -39 -4 3 ½ -15 ppt? Q > Ksp ppt [PO4 ] = (Ksp/[Ba ] ) = [(6 x 10 )/(5 x 10 ) ] = 7 x 10 M 6. 15. 2+ 2- Ksp = [Pb ][SO4 ] 2+ 0.45 L x 0.125 mol = 0.056 mol = 0.080 M 2+ 2- -8 -4 -5 [Ca ] [Pb ] = Ksp/[ SO4 ] = 1.8 x 10 /2 x 10 = 9 x 10 M 1 L 0.7 L 7. 3- 0.25 L x 0.40 mol = 0.10 mol = 0.14 M - 2+ ½ -7 ½ -3 [PO4 ] [F ] = (Ksp/[Ba ]) = (1.8 x 10 /0.0040) = 6.7 x 10 M 1 L 0.7 L - 2+ ½ -7 ½ -2 sp [F ] = (K /[Pb ]) = (7.1 x 10 /0.0060) = 1.1 x 10 M 2+ 3 3- 2 3 2 -5 - -3 Q Q = [Ca ] [PO4 ] = (0.080) (0.14) = 1 x 10 BaF2 will precipitate first when [F ] = 6.7 x 10 M 2. ppt? Q > Ksp ppt 2+ 2- D Copper (II) is Cu and sulfate is SO4 CuSO4 16. 2+ 2- -7 -9 Q = [Sr ][CO3 ] = (0.02)(2 x 10 ) = 4 x 10 3. SrCO3 Q > Ksp ppt Metal (cation) plus nonmetal (anion) does not use 2+ 2- -7 -9 D Q = [Mg ][CO3 ] = (0.02)(2 x 10 ) = 4 x 10 prefixes. The ending for a non-polyatomic ion is ide MgCO3 Q < Ksp no ppt 4. 2+ 3- 17. The precipitate is Pb3(PO4)2(s). Only Pb and PO4 are C 2+ 3- The difference between the two answers illustrates the reactants 3 Pb + 2 PO4 Pb3(PO4)2(s) common ion effect. 5. 18. a. .30 L x .40 mol/L = .12 mol Ba2+ – (.20 L x .20 mol/L = . B 2- 2+ Marble dissolves in acid. 040 mol CO3 ) = 0.080 mol Ba /0.50 L = 0.16 M + 2+ CaCO3 + 2 H Ca + H2O + CO2 6. 3- + b. .1 L x 1 mol/L = .1 mol PO4 + .3 mol Na A + - + Acid absorbed by Mg(OH)2. .1 L x 1 mol/L = .1 mol Ag + .1 mol NO3 (Ag = 0) + 2+ Mg(OH)2 + 2 H Mg + 2 H2O 7. 19. Pb2+ is insoluble in Cl-, All ions are insoluble in S2- and B - 2+ - Name Formula Corrected OH , Cu forms complex with NH3, others form OH ppt. hexammineiron(II) 8. [Fe(NH3)4](SO4)2 [Fe(NH3)6]SO4 + + - All NH4 , K and NO3 salts are soluble. Most sulfate C potassium carbonates are insoluble including BaCO3. tetracyanonickelate(II [Ni(CN)4]K4 K2[Ni(CN)4] 9. + + 2- ) All NH4 and K salts are soluble. Most SO4 salts are A 20. soluble. Mg(OH)2 is insoluble, but dissolves in acid + + 2+ - 2- 10. Ag + 2 NH3 ⇄ Ag(NH3)2 Cu + 4 Cl ⇄ Cu(Cl)4 3+ - - 3+ - 2+ KMnO4 is a common oxidizing agent that is purple and Al + 4 OH Al(OH4) Fe + SCN FeSCN C ⇄ ⇄ turns clear when reduced. (Used in the % peroxide lab) 21. a. 11. Solid AgCl goes into solution as complex ion. 2+ CuSO4 is light blue. Cu compounds vary from yellow- + - B AgCl(s) + 2 NH3(aq) Ag(NH3)2 + Cl blue to green-blue b. 12. Light blue Cu2+ ion changes to dark blue complex. PbSO4 and KCl are white, but KCl is soluble whereas 2+ 2+ (light) Cu + 4 NH3(aq) Cu(NH3)4 (dark) A PbSO4 is insoluble. 22. a. 13. 2+ 2- -13 MnS(s) Mn + S Ksp = 2.5 x 10 2+ 2+ ⇄ Cu + 4 NH3 Cu(NH3)4 + 2- -20 -1 C 2 H + S ⇄ H2 S(aq) K = (1.0 x 10 ) light blue dark blue + 2+ 7 MnS(s) + 2 H ⇄ Mn + H2S(aq) K = 2.5 x 10 14. 2+ b. Hexa = 6, amine = NH3 and iron(II) = Fe . Since the 2+ - A 2- Cu(OH)2 ⇄ Cu + 2 OH Ksp = 4.8 complex has a net charge of +2, it takes 1 SO4 . x 10-20 15. 2+ 2+ 12 2+ - + - Cu + 4 NH3 ⇄ Cu(NH3)4 Kf = 5 x 10 Ni + 2 OH Ni(OH)2(s), Ag + Cl AgCl(s), 2+ - -7 D 2+ 2- + Cu(OH)2(s) + 4 NH3(aq) ⇄ Cu(NH3)4 + 2 OH K = 2.4 x 10 Ba + SO4 BaSO4(s) only Na is spectator. c. 16. 2+ - -7 3+ - 3 BaF2(s) ⇄ Ba + 2 F Ksp = 1.8 x 10 Ksp = [Al ][OH ] + - -4 -2 A 3+ - 3 -31 -4 3 -19 2(H + F ⇄ HF ) Kw = (6.8 x 10 ) [Al ] = Ksp/[OH ] = (2 x 10 )/(1 x 10 ) = 2 x 10 M + 2+ Ba(OH)2(s) + 2 H ⇄ Ba + 2 H2O K = 3.9 17. 3 4 d. Ksp = (s)(3s) = 27s 3+ - -30 D ¼ -31 ¼ Cr(OH)3(s) ⇄ Cr + 3 OH Ksp = 1.6 x 10 s = (Ksp/27) = [(2 x 10 )/27] + - -14 -3 3(H + OH ⇄ H2 O) Kw = (1 x 10 ) 18. + 3+ 12 3 4 Cr(OH)3(s) + 3 H ⇄ Cr + 3 H2O K = 1.6 x 10 Ksp = (s)(3s) = 27s C -6 4 -24 -23 e. Ksp = 27(1.0 x 10 ) = 27 x 10 = 2.7 x 10 3+ - -30 Cr(OH)3(s) Cr + 3 OH Ksp = 1.6 x 10 19. ⇄ 3+ - 3 -8 4 -32 3+ - - 29 Q = [Al ][OH ] = (1 x 10 ) = 1 x 10 < Ksp Cr + 4 OH ⇄ Cr(OH)4 Kf = 8 x 10 C - - no precipitate Cr(OH)3(s) + OH ⇄ Cr(OH)4 K = 1.28 f. 20. 3+ - -31 The property is amphoterism. Al(OH)3(s) ⇄ Al + 3 OH Ksp = 2.0 x 10 B 3+ - - 33 Al + 4 OH ⇄ Al(OH)4 Kf = 1.0 x 10 21. Practice Multiple Choice 2+ 2- -15 MnS(s) ⇄ Mn + S Ksp = 5 x 10 D + 2- -20 -1 1. 2 H + S ⇄ H2S K = (1.0 x 10 ) - - - perchlorate = ClO4 , chlorate = ClO3 , chlorite = ClO2 22. A - + - 2 and hypochorite = ClO Ksp = [Cu ][I ] = (s)(s) = s (where s = solubility) C 2 -6 2 -12 Ksp = s = (2 x 10 ) = 4 x 10 23. i. + 2 2- 2 3 2+ - -11 Ksp = [Ag ] [CrO4 ] = (2s) (s) = 4s (where s = solubility) Ca(OH)2 ⇄ Ca + 2 OH Ksp = 1.8 x 10 D ⅓ -12 ⅓ + - 14 -2 s = (Ksp/4) = (8/4 x 10 ) 2 H + 2 OH ⇄ 2 H2 O K = (1 x 10 ) + 2+ 17 24. Ca(OH)2 + 2 H ⇄ Ca + 2 H2O K = 1.8 x 10 2+ - 2 Ksp = [Pb ][F ] j. D - 2+ ½ -8 -6 ½ -2 ½ [F ] = (Ksp/[Pb ]) = [(4 x 10 )/(1 x 10 )] = (4 x 10 ) 2+ - -11 yes Ca(OH)2 ⇄ Ca + 2 OH Ksp = 1.8 x 10 25. 2 HF 2 H+ + 2 F- K = (6.8 x 10-4)2 2+ ⇄ Pb is soluble in hot and forms a precipitate with + - -14 -2 2 H + 2 OH 2 H2 O Kw = (1 x 10 ) B 2- 2+ + ⇄ CrO4 (PbCrO4) Pb . Ag is soluble in NH3 2+ - 10 Ca(OH)2 + 2 HF ⇄ Ca + 2 F + 2 H2O K = 8.3 x 10 Practice Free Response 1. a. (1) - 2+ 2 OH + Pb Pb(OH)2(s) (2) - - 1.0 L OH x 1 mol OH x 1 mol Pb(OH)2 = 0.5 mol 1 L OH- 2 mol OH- b. (1) + 2+ 2 H + CaCO3(s) Ca + H2O + CO2(g) (2) The H+ ions in acid rain react with the marble statues and the soluble compounds of Ca are washed away. 2. a.
CuSO4• 5 H2O (is blue) and Ni(NO3)2•6H2O (is green) b.
CaCO3 (most carbonates are insoluble) and BaSO4 2+ 2+ 2+ 2+ (insoluble sulfates include Hg2 , Pb , Sr and Ba ) c.
Al(NO3)3• 9 H2O (is not a chloride, which forms a white precipitate with Ag+, and is a nitrate, which are soluble) d. NaCl (no water of hydration) e.
Mix solutions of BaCl2 • 2 H2O and CuSO4 • 5 H2O form a precipitate (BaSO4). 3. a. 2+ - 2 2+ - 2 Ksp = [Ca ][OH ] [Ca ] = Ksp/[OH ] [Ca2+] = 1.8 x 10-11/(2.0 x 10-4)2 = 4.5 x 10-4 M b. 2+ - 2 Ksp = [Ca ][OH ] 1.8 x 10-11 = (s)(2s)2 = 4s3 s = 1.65 x 10-4 M c, 1.50 L x 1.65 x 10 -4 mol x 74.1 g = 0.018 g 1 L 1 mol d. 2+ - 2 Ksp = [Ca ][OH ] 1.8 x 10-11 = (s)(1.0 x 10-3)2 s = 1.8 x 10-5 M e. 0.100 L x 1.8 x 10 -5 mol x 74.1 g = 1.3 x 10-4 g 1 L 1 mol f. s = 0.15 g/1 L x 1 mol/74.1 g = 2.0 x 10-3 M 3 -3 3 -8 Ksp = 4s = 4(2.0 x 10 ) = 3.2 x 10 g. [Ca2+]: (0.45 L x 0.0125 mol/1 L)/0.7 L = 0.0080 M [OH-]: (0.25 L x 0.0040 mol/1 L)/0.7 L = 0.0014 M 2+ - 2 2 -8 Q = [Ca ][OH ] = (0.0080)(0.0014) = 1.6 x 10 > Ksp h. - 2+ ½ -11 -3 ½ [OH ] = (Ksp/[Ca ]) = (1.8 x 10 /4.0 x 10 ) - -5 [OH ] = 6.7 x 10 M Ca(OH)2 will precipitate first when [OH-] = 6.7 x 10-5 M - 2+ ½ -12 -4 ½ -4 [OH ] = (Ksp/[Mg ]) = (6.0 x 10 /1.0 x 10 ) = 2.4 x 10 M