NAME ______DATE ______PERIOD 1 2 3 4 5 6 7 Placing Electrons in Atoms WS10072009 page 1
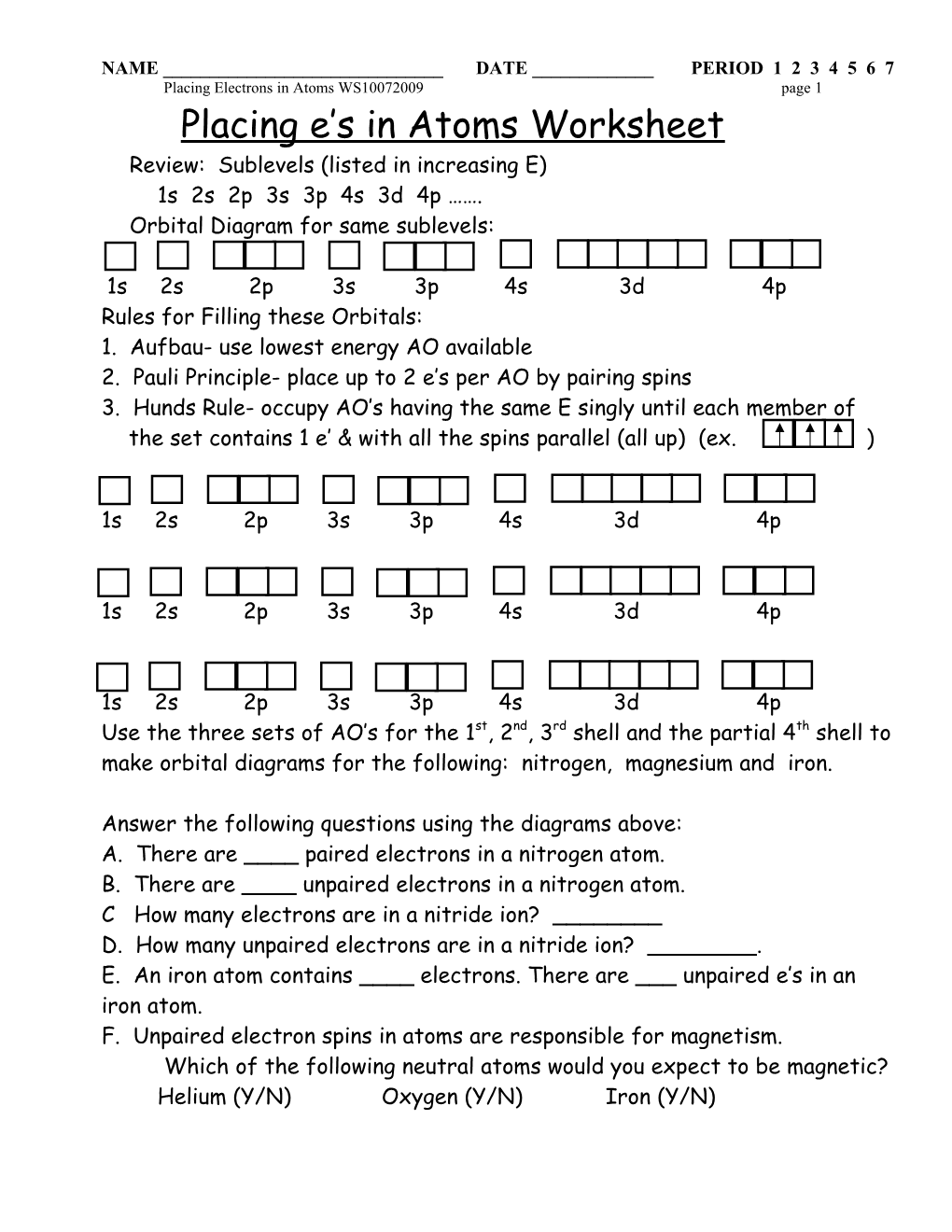
Placing e’s in Atoms Worksheet Review: Sublevels (listed in increasing E) 1s 2s 2p 3s 3p 4s 3d 4p ……. Orbital Diagram for same sublevels:
1s 2s 2p 3s 3p 4s 3d 4p Rules for Filling these Orbitals: 1. Aufbau- use lowest energy AO available 2. Pauli Principle- place up to 2 e’s per AO by pairing spins 3. Hunds Rule- occupy AO’s having the same E singly until each member of the set contains 1 e’ & with all the spins parallel (all up) (ex. )
1s 2s 2p 3s 3p 4s 3d 4p
1s 2s 2p 3s 3p 4s 3d 4p
1s 2s 2p 3s 3p 4s 3d 4p Use the three sets of AO’s for the 1st, 2nd, 3rd shell and the partial 4th shell to make orbital diagrams for the following: nitrogen, magnesium and iron.
Answer the following questions using the diagrams above: A. There are ____ paired electrons in a nitrogen atom. B. There are ____ unpaired electrons in a nitrogen atom. C How many electrons are in a nitride ion? ______D. How many unpaired electrons are in a nitride ion? ______. E. An iron atom contains ____ electrons. There are ___ unpaired e’s in an iron atom. F. Unpaired electron spins in atoms are responsible for magnetism. Which of the following neutral atoms would you expect to be magnetic? Helium (Y/N) Oxygen (Y/N) Iron (Y/N) NAME ______DATE ______PERIOD 1 2 3 4 5 6 7 Placing Electrons in Atoms WS10072009 page 2 Electron Configurations How to Write: 1. List the sublevels in order of increasing energy. 2. Determine the orbital diagram for the atom of interest 3. Use the orbital diagram to determine the number of e’s that occupy each sublevel. 4. Label each sublevel with a superscript number corresponding to the number of e’s in the sublevel. Example: Use your orbital diagram for N to show that the electron configuration 1s2 2s2 2p3 Write electron configurations for the following: A. N B. Mg C. Carbon D. Oxygen E. F F. Fluoride G. Iron H. Mg2+ I. Sulfur J. S2- K. What information is lost when using electron configurations that is clearly shown in a correct orbital diagram?
L. How many unpaired electron spins are in: (a) 1s22s22p4 (a) 1s22s22p63s23p64s1 Valence e’s & Dot Structures: Valence electrons – e’s in outermost orbitals; responsible for chemical properties Example: Carbon 1s22s22p2 ; the outermost AO’s are 2s & 2p containing 4 valence e’s How many valence electrons are there in: (a) beryllium (b) magnesium (c) sulfur (d) potassium
Dot Structures – valence e’s are shown as dots written around the element’s chemical symbol. Rules: Place dots one at a time first filling out the sides of an imaginary square around the symbol. Do not pair dots until each side of the square has one dot. If more dots are needed start pairing them until you reach a maximum of 8 valence e’s (4 pairs).
Example: Silicon is Si and N is N 4 VE’s and 5 VE’s
Write Electron Dot Structures for: A. Beryllium B. Calcium
C. Fluorine D. Chlorine
E. Argon F. Neon
G. Sulfur H. Phosphorus