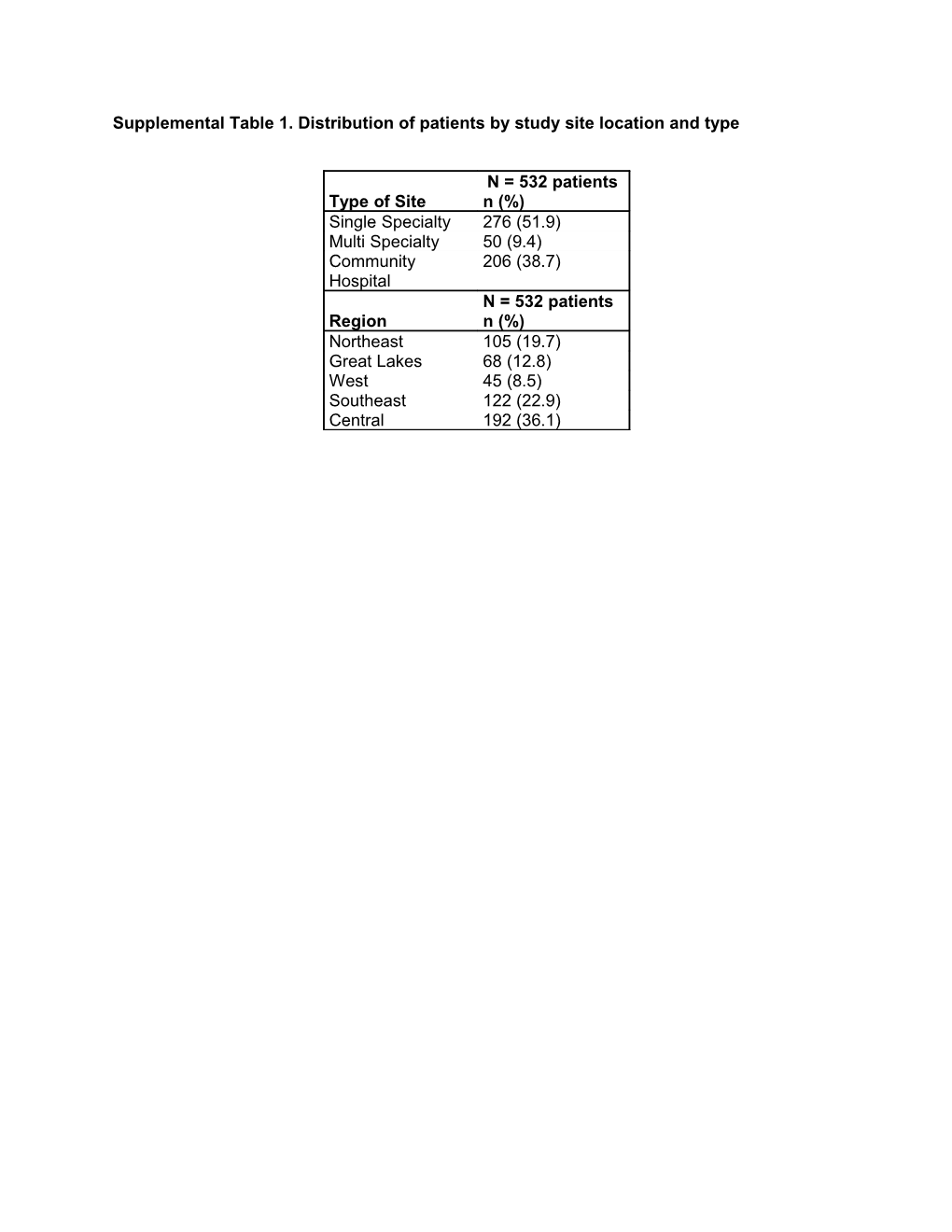
Supplemental Table 1. Distribution of patients by study site location and type
N = 532 patients Type of Site n (%) Single Specialty 276 (51.9) Multi Specialty 50 (9.4) Community 206 (38.7) Hospital N = 532 patients Region n (%) Northeast 105 (19.7) Great Lakes 68 (12.8) West 45 (8.5) Southeast 122 (22.9) Central 192 (36.1) Supplemental Table 2. Chemotherapy regimens
Regimen N (%) Dosing and schedule Standard Planned Cycles References cycles cycles receiv Median ed (range) Median (range)
TCa 221 (41.5) 14- or 21-day Docetaxel at 75 mg/m2 4 4 (4–6) 4 (1–6) [1,2] with cyclophosphamide at 600 mg/m2 ACa 58 (10.9) 14- or 21-day doxorubicin at 60 mg/m2 4 4 (4–6) 4 (4–6) [2,3] with cyclophosphamide at 600 mg/m2 TCH 56 (10.5) 21-day docetaxel at 75 mg/m2 with 6 6 (6–6) 6 (2–6) [2] carboplatin at planned dose amount, followed trastuzumab TACa 34 (6.4) 14- or 21-day docetaxel at 75 mg/m2 6 6 (4–6) 6 (1–6) [2,4] with doxorubicin at 50 mg/m2 and cyclophosphamide at 500 mg/m2 b AC-Tweekly 22 (4.1) 21-day AC followed by weekly 4 AC 8 (8–8) 8 (3–8) [2,5-7] paclitaxel at 80 mg/m2 4 T AC-T14 83 (15.6) 14-day AC followed by 14-day 4 AC 8 (8–12) 8 (5–12) [2,5,6,8] paclitaxel at 175 mg/m2 4 T AC14- 54 (10.2) 14-day AC followed by weekly 4 AC 8 (7–10) 8 (4–10) [5-8] b,c 2 Tweekly paclitaxel at 80 mg/m 4 T AC-T 4 (0.8) 21-day AC followed by 21-day 4 AC 8 (8–8) 8 (8–8) [2,5,6] paclitaxel at 175 mg/m2 4 T a 14-day regimens were allowed as many physicians have adopted dose-dense regimens b One cycle of paclitaxel was defined as 3 weekly administrations given on days 1, 8, and 15 c Regimen included because many physicians have adopted weekly taxanes Supplemental Table 3. Neutropenia by cycle
Grade 3/4 Grade 3/4 FN Grade 4 FN neutropenia n (%) n (%) n (%) Overall 215 (40.4) 18 (3.4) 12 (2.3) (N = 532) Cycle 1 144 (27.1) 5 (0.9) 4 (0.8) (N = 532) Cycle 2 104 (19.7) 4 (0.8) 3 (0.6) (N = 527) Cycle 3 96 (18.4) 7 (1.3) 5 (1.0) (N = 522) Cycle 4 100 (19.4) 3 (0.6) 2 (0.4) (N = 516) Cycle 5 19 (6.6) 1 (0.3) 0 (0) (N = 287) Cycle 6 22 (7.9) 0 (0) 0 (0) (N = 278) Cycle 7 3 (1.9) 0 (0) 0 (0) (N = 155) Cycle 8 3 (2.0) 0 (0) 0 (0) (N = 148) FN febrile neutropenia Supplemental Table 4. G-CSF use by chemotherapy regimen
Any G-CSF Primary prophylactic G-CSFa Regimen n (%) n (%) TC 196 (88.7) 144 (73.5) AC 50 (86.2) 46 (92.0) TCH 52 (92.9) 42 (80.8) TAC 34 (100.0) 34 (100.0)
AC-Tweekly 10 (45.5) 2 (20.0) AC-T14 83 (100.0) 83 (100.0)
AC14-Tweekly 53 (98.1) 50 (94.3) AC-T 3 (75.0) 3 (100.0) G-CSF granulocyte colony-stimulating factor, TC docetaxel + cyclophosphamide, AC doxorubicin + cyclophosphamide, TCH docetaxel + carboplatin + trastuzumab, TAC docetaxel+ doxorubicin + cyclophosphamide, AC-Tweekly 21-day AC followed by weekly paclitaxel, AC-T14
14-day AC followed by 14-day paclitaxel, AC14-Tweekly 14-day AC followed by weekly paclitaxel,
AC-T 21-day AC followed by 21-day paclitaxel a Relative to patients who received G-CSF at some point Supplemental references
1. Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, Pippen JE, Bordelon JH, Kirby RL, Sandbach J, Hyman WJ, Richards DA, Mennel RG, Boehm KA, Meyer WG, Asmar L, Mackey D, Riedel S, Muss H, Savin MA (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-Year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27 (8):1177-1183 2. NCCN Breast Cancer. NCCN Clinical Practice Guidelines in Oncology v3.2013 3. Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, Bowman D, Wolmark N, Wickerham DL, Kardinal CG, et al. (1990) Two months of doxorubicin- cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8 (9):1483-1496 4. Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al- Tweigeri T, Chap L, Juhos E, Guevin R, Howell A, Fornander T, Hainsworth J, Coleman R, Vinholes J, Modiano M, Pinter T, Tang SC, Colwell B, Prady C, Provencher L, Walde D, Rodriguez-Lescure A, Hugh J, Loret C, Rupin M, Blitz S, Jacobs P, Murawsky M, Riva A, Vogel C (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352 (22):2302-2313. doi:10.1056/NEJMoa043681 5. Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E, 3rd, Schilsky RL, Wood WC, Muss HB, Norton L (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21 (6):976-983 6. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G, Soran A, Wolmark N (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23 (16):3686-3696. doi:10.1200/JCO.2005.10.517 7. Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr., Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358 (16):1663-1671. doi:10.1056/NEJMoa0707056 8. Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L (2003) Randomized trial of dose- dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21 (8):1431-1439