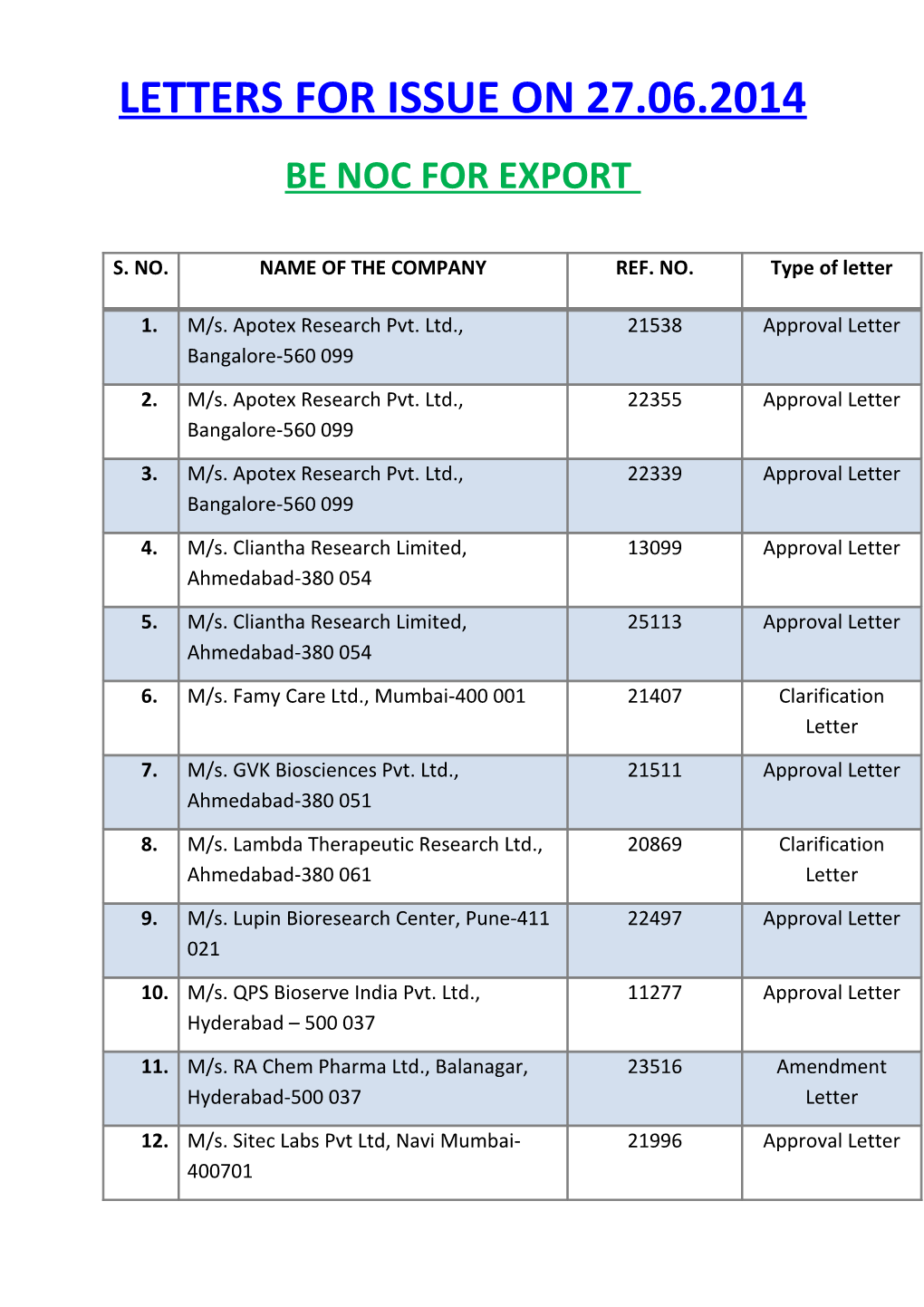
LETTERS FOR ISSUE ON 27.06.2014 BE NOC FOR EXPORT
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Apotex Research Pvt. Ltd., 21538 Approval Letter Bangalore-560 099
2. M/s. Apotex Research Pvt. Ltd., 22355 Approval Letter Bangalore-560 099
3. M/s. Apotex Research Pvt. Ltd., 22339 Approval Letter Bangalore-560 099
4. M/s. Cliantha Research Limited, 13099 Approval Letter Ahmedabad-380 054
5. M/s. Cliantha Research Limited, 25113 Approval Letter Ahmedabad-380 054
6. M/s. Famy Care Ltd., Mumbai-400 001 21407 Clarification Letter
7. M/s. GVK Biosciences Pvt. Ltd., 21511 Approval Letter Ahmedabad-380 051
8. M/s. Lambda Therapeutic Research Ltd., 20869 Clarification Ahmedabad-380 061 Letter
9. M/s. Lupin Bioresearch Center, Pune-411 22497 Approval Letter 021
10. M/s. QPS Bioserve India Pvt. Ltd., 11277 Approval Letter Hyderabad – 500 037
11. M/s. RA Chem Pharma Ltd., Balanagar, 23516 Amendment Hyderabad-500 037 Letter
12. M/s. Sitec Labs Pvt Ltd, Navi Mumbai- 21996 Approval Letter 400701 13. M/s. Sitec Labs Pvt Ltd, Navi Mumbai- 23046 Approval Letter 400701
14. M/s. Torrent Pharmaceuticals Ltd. 21493 Approval Letter (Research Centre), Gujarat
ONLY TEST LICENCE FOR BE
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Accutest Research Laboratories (I) Pvt. 22427 Test Licence Ltd., Navi Mumbai-400 709
2. M/s. Apotex Research Pvt. Ltd., Bangalore-560 21537 Test Licence 099
3. M/s. Azidus Laboratories Ltd., Chennai-600 048 23150 Test Licence
4. M/s. Azidus Laboratories Ltd., Chennai-600 048 23149 Test Licence
5. M/s. Lambda Therapeutic Research Ltd., 22553 Test Licence Ahmedabad-380 061
6. M/s. Unichem Laboratories Ltd., Mumbai-400 21555 Test Licence 102
TEST LICENCES
S. No. Name of the Company Diary No. Type of Letter
1. AET Laboratories Ltd 23525, Approval Letter 34700
2. Agila Specialties Pvt. Ltd 23387, Approval Letter 34772
3. Agila Specialties Pvt. Ltd 23388, Approval Letter 34771
4. Alkem Laboratories Ltd 23482, Approval Letter 34908
5. BDR Pharmaceuticals 23340, Approval Letter International Pvt. Ltd 34896
6. Cadila Pharmaceutical Limited 23361, Approval Letter 34892
7. Centaur Pharmaceuticals Pvt 23526, Approval Letter Ltd 34726
8. Cipla Ltd 23480, Approval Letter 34709
9. Cipla Ltd 23481, Approval Letter 34707
10. Glenmark Pharmaceuticals Ltd 23537, Approval Letter 34696
11. Glenmark Pharmaceuticals Ltd 23539, Approval Letter 34695
12. Johnson and Johnson Ltd 23389, Approval Letter 34762
13. Johnson and Johnson Ltd 23390, Approval Letter 34761
14. Macleods Pharmaceuticals Ltd 23333, Approval Letter 34884
15. Macleods Pharmaceuticals Ltd 23334, Approval Letter 34883
16. Macleods Pharmaceuticals Ltd 23335, Approval Letter 34882
17. Onco Therapies Ltd 23386, Approval Letter 34887
18. Sharon BioMedicine Ltd 23524, Approval Letter 34701 19. Sun Pharmaceutical Industries 23336, Approval Letter Ltd 34905
20. Sun Pharmaceutical Industries 23337, Approval Letter Ltd 34903
21. Sun Pharmaceutical Industries 23338, Approval Letter Ltd 34909
22. Tevapharm India Pvt Ltd 23503, Approval Letter 34894
23. Tevapharm India Pvt Ltd 23504, Approval Letter 34898
24. Tevapharm India Pvt Ltd 23505, Approval Letter 34899
25. Tevapharm India Pvt Ltd 23506, Approval Letter 34889
26. Tevapharm India Pvt Ltd 23507, Approval Letter 34888
27. Tevapharm India Pvt Ltd 23508, Approval Letter 34886
28. Tevapharm India Pvt Ltd 23509, Approval Letter 34893
29. Tevapharm India Pvt Ltd 23510, Approval Letter 34891
30. Tevapharm India Pvt Ltd 23511, Approval Letter 34811
31. Tevapharm India Pvt Ltd 23512, Approval Letter 34806
32. Tevapharm India Pvt Ltd 23513, Approval Letter 34800
33. Tevapharm India Pvt Ltd 23514, Approval Letter 34796
34. Tevapharm India Pvt Ltd 23515 Approval Letter 35. Tevapharm India Pvt Ltd 23527, Approval Letter 34816
36. Tevapharm India Pvt Ltd 23528, Approval Letter 34815
37. Tevapharm India Pvt Ltd 23529, Approval Letter 34814
38. Tevapharm India Pvt Ltd 23530, Approval Letter 34813
39. Wockhardt Ltd 23327, Approval Letter 34653
40. Wockhardt Ltd 23328, Approval Letter 34875
41. Wockhardt Ltd 23329, Approval Letter 34877
42. Wockhardt Ltd 23330, Approval Letter 34878
43. Wockhardt Ltd 23331, Approval Letter 34881
44. Wockhardt Ltd 23332, Approval Letter 34880
45. Wockhardt Ltd 23355, Approval Letter 34873
46. Wockhardt Ltd 23356, Approval Letter 34879
47. Wockhardt Ltd 23357, Approval Letter 34876
FDC DIVISION
S.NO NAME OF COMPANY DIARY DOCUMENT . NO 1. M/s. Torrent Pharmaceuticals Pvt. Ltd., 15116 Letter
2. M/s. Biodeal Pharmaceuticals Pvt. Ltd., 82926 Clarification Letter
3. M/s. Olive Healthcare 41615 Clarification Letter
4. M/s. Bajaj Healthcare Ltd., 26113 Clarification Letter
5. M/s. Indi Pharma Pvt. Ltd., 41774 Clarification Letter
NEW DRUG
S.NO. NAME OF APPLICANT DIARY NO. TYPE OF LETTER 1. M/s. MSN Labs - Approval Letter
2. M/s. Akums Drugs - Clarification Letter
MEDICAL DEVICE
S.NO. COMPANY NAME DIARY NO STATUS
1. M/s. Lepu Medical Technology (Beijing) Co. Ltd. 23484 Approval (F-RC)
2. M/s. Covidien Healthcare India Pvt. Ltd. 5425 Approval (E-RC)
3. M/s. Biotronik Medical Devices India Pvt. Ltd. 18129 Approval (R-IL)
4. M/s. Meril Healthcare Pvt. Ltd. 19448 Approval (R-IL)
5. M/s. Biomed Orthopaedics India Pvt. Ltd. 19452 Approval (E-IL)
6. M/s. Apothecaries Pvt. Ltd. 16777 Approval Letter
7. The State Drugs Controller, Haryana (CLAA) FTS-32263 Approval Letter
The Deputy Drugs Controller (I), Ghaziabad (CLAA)
(M/s. La-Med Healthcare Pvt. Ltd.)
DIAGNOSTIC DIVISION
S.NO. COMPANY NAME DIARY NO STATUS
1. M/s. Euro Diagnostics Systems Pvt. Ltd. 18957 Approval (IL)
2. M/s. Globe Bio-Medicals. 19113 Approval (IL)
3. M/s. Randox Laboratories (India) Pvt. Ltd. 20330 Approval (E-IL)
VACCINE DIVISION
S. No. COMPANY NAME Dy.No. STATUS
1 M/s GSK Pharmaceuticals ltd 11284, 12197 Approval Letter
2 M/s Sanofi Pasteur India Pvt, 12443 Approval Letter Ltd
3 M/s Stallen South Asia Pvt, 20211, Approval Letter ltd
4 M/s EliLilly and company 18716 Clarification Letter (india) Pvt, ltd
5 M/s Bharat Biotech 7622 Approval Letter International ltd
6 M/s Intas Pharmaceuticals 18368 Clarification Letter Ltd
7 M/s Roche Product India Pvt, 15475 Clarification Letter ltd 8 M/s Shantha Bioethics Ltd Clarification Letter
BIOLOGICAL DIVISION
S. No. COMPANY NAME Dy.No. STATUS
1 M/s Biocon ltd 22331, 12995 (fts Approval, Clarification no) Letter,
2 M/s Mabpharma ltd 21903 Clarification Letter
COSMETICS DIVISION
S.N COMPANY NAME DIARY NO STATUS O.
1. M/s. Wockhardt Limited, Mumbai 15932 Deficiency Letter
2. M/s. New Beauty Center, Mumbai 17456 Deficiency Letter
3. M/s. Priss Trading Company, Ernakulum 13701 Approval Letter
4. M/s. Hindustan Uniliver Limited, Mumbai 18448 Approval Letter