Physics of X-Ray Fluorescence
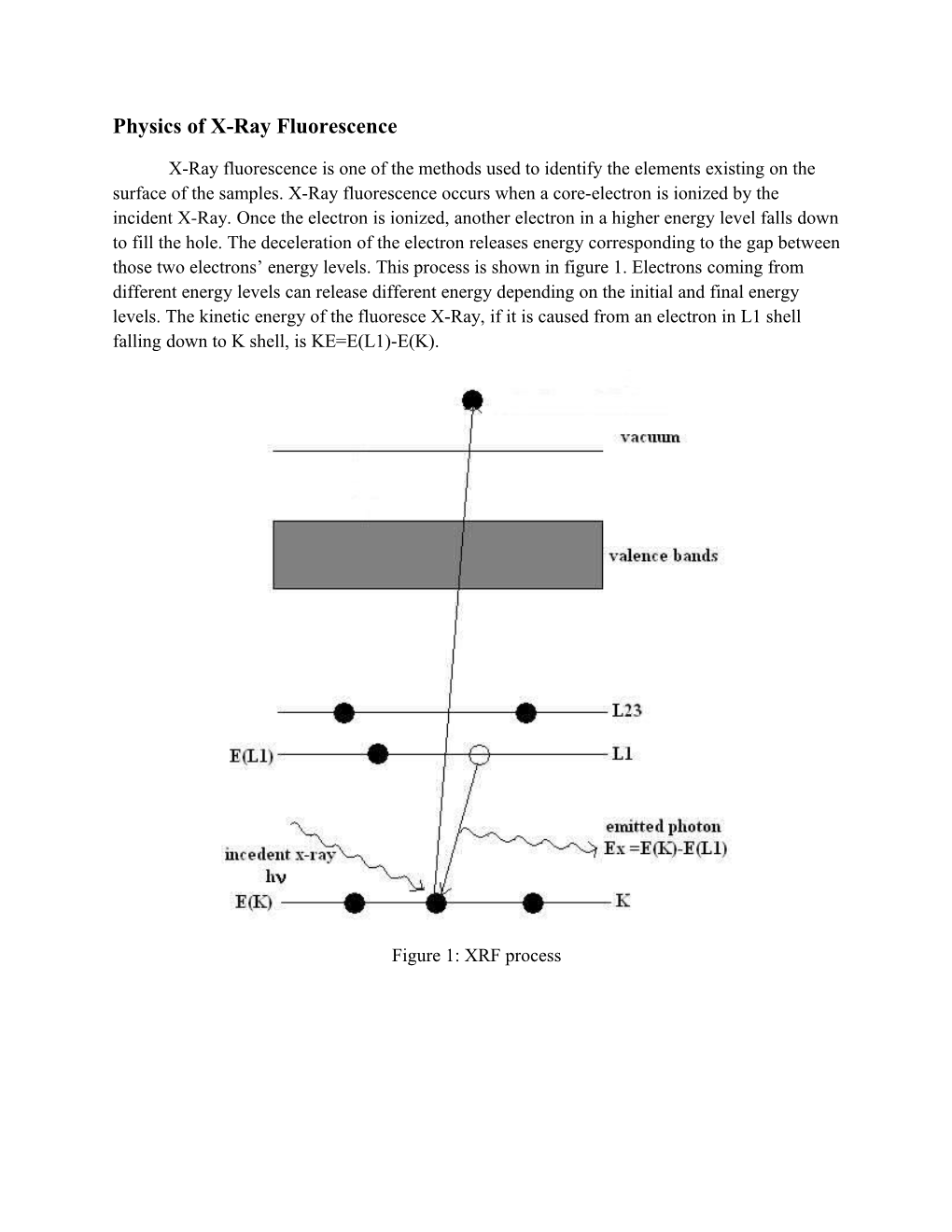
X-Ray fluorescence is one of the methods used to identify the elements existing on the surface of the samples. X-Ray fluorescence occurs when a core-electron is ionized by the incident X-Ray. Once the electron is ionized, another electron in a higher energy level falls down to fill the hole. The deceleration of the electron releases energy corresponding to the gap between those two electrons’ energy levels. This process is shown in figure 1. Electrons coming from different energy levels can release different energy depending on the initial and final energy levels. The kinetic energy of the fluoresce X-Ray, if it is caused from an electron in L1 shell falling down to K shell, is KE=E(L1)-E(K).
Figure 1: XRF process Example of using XRF
Experiment
1. Use X-Met 3000T to measure three samples on the back sides, machine was operated at 40 keV and 7µm in wavelength (λ)
2. Analyze obtained data using PyMCA software
2.1 Open the data file
2.2 Calibrate the energy scale using AlKL3 as a reference line
2.3 Fit the calibrated curve with the created configuration file using the machine operating data
2.4 Export the fitted data to the html format
2.5 Use six most fitted elements to find the relationship between samples by plotting graphs of two elements in samples for all of the selected elements
Results
From fitted curves of the three samples, we found that there were six most fitted elements existing in all of the samples. They were titanium (Ti), Chromium (Cr), Manganese (Mn), Iron in L and L3 (Fe), Cobalt (Co), and Nickel (Ni). Figure 2A-2C shows the measured curves after fitting; a black line is the measured curve, a red line is fitted curve, a green line is a continuum curve, and a pink line is a pile up curve. Figure 3A-3O are the graphs between existing elements from the three samples using mass fraction given in the concentration table obtained from the software; a red dot is 15018J-back, a green dot is 17157T-back, and a blue dot is 12363H-back. Table 1-3 are parameter used to fit curves from the sample named 15018J-back, 17157T-back, and 12363H-back, respectively. Table 4 is the six selected elements’ mass fraction used to plot figure 5-19. Picture 1-3 are samples used in this experiment, and picture 4 is the workers’ photo. Figure 2A: Curves of sample 15018J-back Figure 2B: Curves of sample 17157T-back Figure 2C: Curves of sample 12363H-back Figure 3A: Graph of Ti versus Cr Figure 3B: Graph of Ti versus Mn
Figure 3C: Graph of Ti versus Fe(L+L3) Figure 3D: Graph of Ti versus Co
Figure 3E: Graph of Ti versus Ni Figure 3F: Graph of Cr versus Mn Figure 3G: Graph of Cr versus Fe(L+L3) Figure 3H: Graph of Cr versus Co
Figure 3I: Graph of Cr versus Ni Figure 3J: Graph of Mn versus Fe(L+L3) Figure 3K: Graph of Mn versus Co Figure 3L: Graph of Mn versus Ni Figure 3M: Graph of Fe(L+L3) versus Co Figure 3N: Graph of Fe(L+L3) versus Ni
Figure 3O: Graph of Zr versus Rh Table 1: Fit, Calibration, Peak shape and Continuum parameter of 15018J-back sample
FIT Calibration parameters Peak shape parameters Continuum parameters parameters Region of Fit 25 - 1535 Zero -3.81757E-002 ST AreaR 1.00000E-001 Type SNIP +/- 7.21550E- +/- 1.00000E- Backgroun 004 001 d Number of 8 Gain 2.00425E-002 ST SlopeR 1.50000E-001 SNIP 30.00000 iterations +/- 1.68621E- +/- 1.50000E- width 006 001 Chi square 14.1785 Noise 9.95176E-002 LT AreaR 0.00000E+000 Smoot 1 +/- 1.69885E- +/- hing 003 0.00000E+000 Filter Width Last Chi 0.0995 % Fano 2.21788E-001 LT SlopeR 1.00000E+001 square +/- 5.51135E- +/- difference 003 1.00000E+001 Sum 1.99929E-008 STEP HeightR 0.00000E+000 +/- 2.84301E- +/- 008 0.00000E+000
Table 2: Fit, Calibration, Peak shape and Continuum parameter of 17157T-back sample
FIT parameters Calibration parameters Peak shape parameters Continuum parameters Region of Fit 25 - 1535 Zero -3.62083E-002 ST AreaR 1.00000E-001 Type SNIP +/- 7.12178E- +/- 1.00000E- Backgroun 004 001 d Number of 9 Gain 2.00311E-002 ST SlopeR 1.50000E-001 SNIP 30.00000 iterations +/- 1.57772E- +/- 1.50000E- width 006 001 Chi square 13.9379 Noise 9.20179E-002 LT AreaR 0.00000E+000 Smoot 1 +/- 3.44525E- +/- hing 003 0.00000E+000 Filter Width Last Chi 0.0413 % Fano 2.26322E-001 LT SlopeR 1.00000E+001 square +/- 2.66517E- +/- difference 002 1.00000E+001 Sum 1.19174E-008 STEP HeightR 0.00000E+000 +/- 2.05539E- +/- 008 0.00000E+000 Table 3: Fit, Calibration, Peak shape and Continuum parameter of 12363H-back sample
FIT parameters Calibration parameters Peak shape parameters Continuum parameters Region of Fit 25 - 1535 Zero -3.00741E-002 ST AreaR 1.00000E-001 Type SNIP +/- 6.84398E- +/- 1.00000E- Backgroun 004 001 d Number of 14 Gain 2.00159E-002 ST SlopeR 1.50000E-001 SNIP 30.00000 iterations +/- 1.57249E- +/- 1.50000E- width 006 001 Chi square 12.8393 Noise 1.06558E-001 LT AreaR 0.00000E+000 Smoot 1 +/- 2.93372E- +/- hing 003 0.00000E+000 Filter Width Last Chi 0.0100 % Fano 2.02731E-001 LT SlopeR 1.00000E+001 square +/- 6.47435E- +/- difference 003 1.00000E+001 Sum 1.99241E-008 STEP HeightR 0.00000E+000 +/- 3.89448E- +/- 008 0.00000E+000
Table 4: Mass fraction for the six selected elements used to plot figure 5-19.
Sample Ti Cr Mn Fe(L+L3) Co Ni 12363H- back 5.68E+05 2.91E+04 882 316.17 8.729 1.401 15018J- back 3.19E+05 6.46E+04 613.3 308.82 11.98 1.407 17157T- back 5.50E+05 1.15E+05 592.4 237.64 9.648 1.424 Picture 1: 17157T- back sample Picture 2: 12363H-back sample
Picture 3: 15018J-back sample Picture 4: Me and my co-worker Kiran vajrapu
Conclusion
From the graphs of elements in the samples, the samples have some elements that can be grouped together but not every element from the same sample can do that. It is hard to tell that those samples have relationships.