Unit: Atomic Theory Chemistry, Wilson Models of the Atom
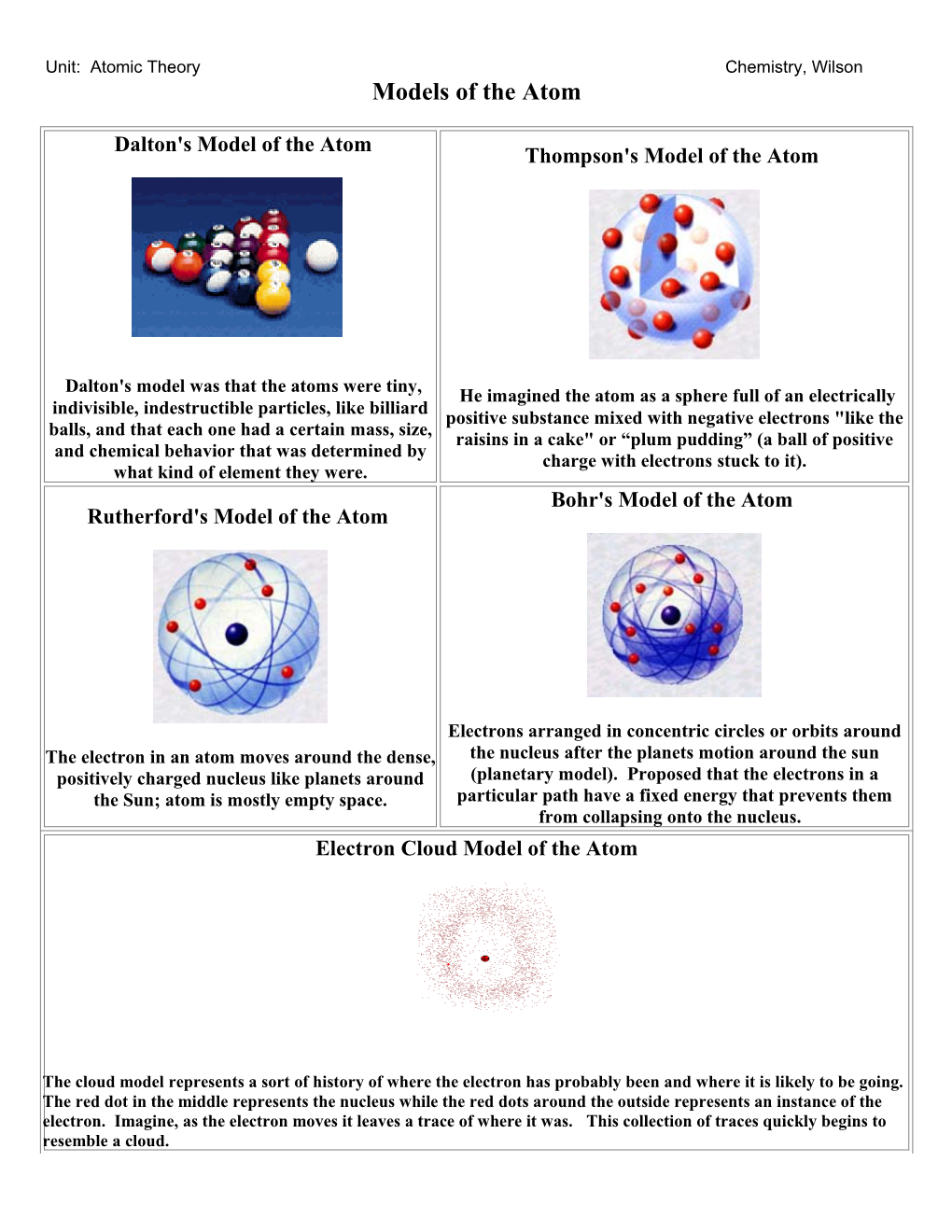
Dalton's Model of the Atom Thompson's Model of the Atom
Dalton's model was that the atoms were tiny, He imagined the atom as a sphere full of an electrically indivisible, indestructible particles, like billiard positive substance mixed with negative electrons "like the balls, and that each one had a certain mass, size, raisins in a cake" or “plum pudding” (a ball of positive and chemical behavior that was determined by charge with electrons stuck to it). what kind of element they were. Bohr's Model of the Atom Rutherford's Model of the Atom
Electrons arranged in concentric circles or orbits around The electron in an atom moves around the dense, the nucleus after the planets motion around the sun positively charged nucleus like planets around (planetary model). Proposed that the electrons in a the Sun; atom is mostly empty space. particular path have a fixed energy that prevents them from collapsing onto the nucleus. Electron Cloud Model of the Atom
The cloud model represents a sort of history of where the electron has probably been and where it is likely to be going. The red dot in the middle represents the nucleus while the red dots around the outside represents an instance of the electron. Imagine, as the electron moves it leaves a trace of where it was. This collection of traces quickly begins to resemble a cloud. Unit: Atomic Theory Chemistry, Wilson
Hog Hilton Unit: Atomic Theory Chemistry, Wilson Hog Hilton You are the manager of a prestigious new hotel in downtown Milwaukee ”Hog Hilton”. It’s just the “snort of the town” and you want to keep its reputation a cut above all the other hotels. Your problem is your clientele. They are hogs in the truest sense.
Your major task is to fill rooms in your hotel. The funny shape of your hotel is to accommodate the habits of the hogs. The penthouse is on the first floor and the less desirable rooms are on the top floor. You must fill up your hotel keeping the following rules in mind:
1. Hogs are lazy and want the best accommodations possible without having to expend energy (they don’t want to climb steps).
2. Hogs can’t stand each other except when rule #1 forces them to put up with each other.
3. If hogs are in the same room they will face in opposite directions.
4. They stink, so you can’t put more than two hogs in each room.
Your hotel looks like the diagram below: ( ___ Represents 1 Room) 9th Floor ___ 8th Floor ______7th Floor ______6th Floor ___ 5th Floor ______4th Floor ___ 3rd Floor ______2nd Floor ___ 1st Floor ___ Your hotel can hold 36 hogs. Sample problems (1) Book 15 hogs into their rooms
(2) Book 25 hogs into their rooms
Class work: On your own, fill your hotel for the following days of the week: Monday: 5 hogs Tuesday: 8 hogs Wednesday: 1 hog Thursday: 12 hogs Friday: 23 hogs Saturday: 18 hogs Sunday: 32hogs Unit: Atomic Theory Chemistry, Wilson On the last page you learned how to fill up an imaginary hotel. Now you will relate this example to electron orbitals. Electron orbitals are modeled by the picture below and are grouped into principal energy levels. 5s ___ n = 4 4p ______n= 4 3d ______n=3 4s ____ n=3 3p ______n=3 3s ___ n=3 2p ______n=2 2s ___ n=2 1s ___ n=1
Questions to think about: (1) Compare this with the Hog Hilton. What are the similarities and the differences?
(2) To go between floors on the Hog Hilton did the hogs need to use energy? Would electrons need to use energy to go between orbitals?
(3) If only ½ the energy necessary to go between the 1s and 2s orbital was available, will an electron go to the 2s orbital?
Examples on how to fill electron orbitals: (1) 7 electrons 3d ______n=3 (4s ____) n=3 3p ______n=3 3s ___ n=3 2p ______n=2 2s ___ n=2 1s ___ n=1 (2) 16 electrons 3d ______n=3 (4s ____) n=3 3p ______n=3 3s ___ n=3 2p ______n=2 2s ___ n=2 1s ___ n=1 Unit: Atomic Theory Chemistry, Wilson
Electron Configuration Practice
Complete an electron orbital diagram for the following numbers of electrons: 10, 24, 13, 3, 5, 26, 22, 17
10 electrons ______
24 electrons ______
13 electrons ______
3 electrons ______
5 electrons ______
26 electrons ______
22 electrons ______
17 electrons ______