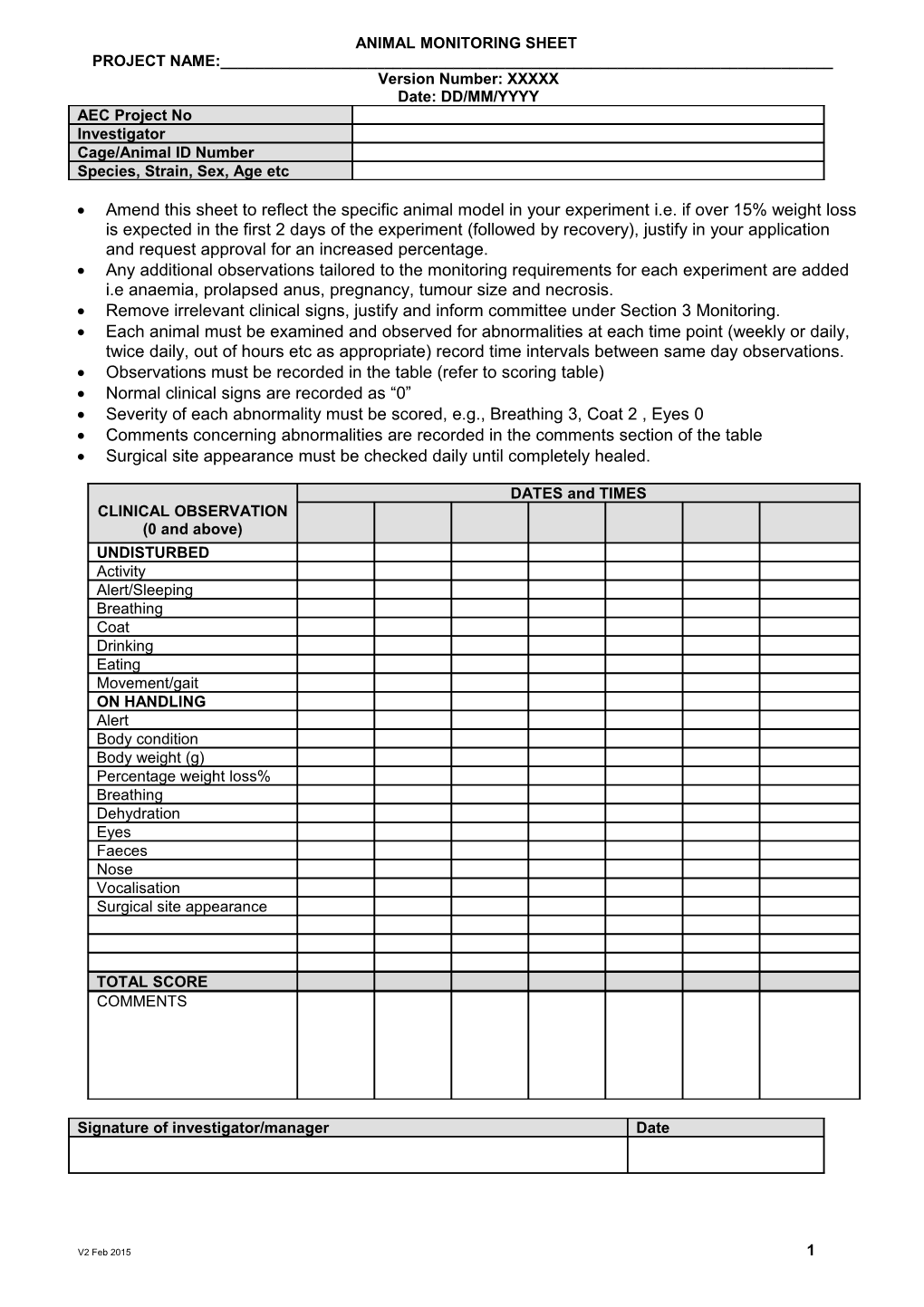
ANIMAL MONITORING SHEET PROJECT NAME:______Version Number: XXXXX Date: DD/MM/YYYY AEC Project No Investigator Cage/Animal ID Number Species, Strain, Sex, Age etc
Amend this sheet to reflect the specific animal model in your experiment i.e. if over 15% weight loss is expected in the first 2 days of the experiment (followed by recovery), justify in your application and request approval for an increased percentage. Any additional observations tailored to the monitoring requirements for each experiment are added i.e anaemia, prolapsed anus, pregnancy, tumour size and necrosis. Remove irrelevant clinical signs, justify and inform committee under Section 3 Monitoring. Each animal must be examined and observed for abnormalities at each time point (weekly or daily, twice daily, out of hours etc as appropriate) record time intervals between same day observations. Observations must be recorded in the table (refer to scoring table) Normal clinical signs are recorded as “0” Severity of each abnormality must be scored, e.g., Breathing 3, Coat 2 , Eyes 0 Comments concerning abnormalities are recorded in the comments section of the table Surgical site appearance must be checked daily until completely healed.
DATES and TIMES CLINICAL OBSERVATION (0 and above) UNDISTURBED Activity Alert/Sleeping Breathing Coat Drinking Eating Movement/gait ON HANDLING Alert Body condition Body weight (g) Percentage weight loss% Breathing Dehydration Eyes Faeces Nose Vocalisation Surgical site appearance
TOTAL SCORE COMMENTS
Signature of investigator/manager Date
V2 Feb 2015 1
CLINICAL SIGNS SEVERITY SCORE – Category I (1-3)
SIGNS 0 1 2 3
Activity normal isolated, huddled/inactive, moribund abnormal isloated OR fitting posture OR overactive Alertness/Sleeping normal dull or little response to unconscious depressed handling Coat normal coat rough unkempt; superficial bleeding or infected wounds, or wounds, hair thinning, severe hairloss, severe pilo- slight pilo-erection erection or self mutilation Faeces normal faeces loose, soiled perineum Presence of diarrhoea for 48 moist hours OR no faeces for 48 hrs OR presence of blood on faeces Movement/ gait normal slight OR walking on tiptoe OR staggering OR limb dragging OR abnormal reluctance to move paralysis gait Nose normal wetness discharge Obstruction of nasal passages or constant purulent discharge Vocalisation normal squeaks struggles and squeaks persistent and repetitive when loudly in response to vocalisation without handling palpated handling of a body part & signs of aggression Surgical site normal mild presence of discharge redness and swelling of the appearance (no redness, and presence of wound and/or separation of swelling mild moderate swelling, wound edges. *Euthanasia if re- or swelling of slight wound suturing and/or surgical redness) wound dehiscence. intervention fails Other
CLINICAL SIGNS SEVERITY SCORE – Category II (4-6)
SIGNS 0 4 5 6
Body condition normal thin loss of body fat, loss of muscle mass that failure to grow enables palpation of the spine Body weight normal reduced Chronic weight loss acute weight loss>10% * weight and growth rate >10%** OR failure chronic weight loss 15% ** OR growth rate to grow failure to grow for juveniles or failure to stabilise body weight Breathing normal rapid, shallow rapid, abdominal laboured, irregular, or gaping breathing mouth breaking, and/or blue skin Dehydration none skin less skin tenting/ sunken skin tenting & eyes and elastic abdomen abdomen sunken Drinking normal increased OR increased OR constantly drinking OR not decreased decreased intake drinking over 24 hours intake over 24 over 48 hours hrs Eating normal increased OR increased OR obese OR not eating over 48 decreased decreased intake hours (amend for obese strains) intake over 24 over 48 hours hours Eyes normal wetness or discharge Severe eye infection with vision dullness obstructed and signs of pain. Other
*, Acute refers to the weight loss occurring within 2 days. **, Chronic refers to the weight loss occurring within 7 days;
V2 Feb 2015 2
SPECIAL HUSBANDRY REQUIREMENTS Record specific diets and supplements, therapies, bedding enrichment or particular food supplements or enrichment that is not appropriate i.e sunflower seeds, paper etc
EUTHANASIA/HUMANE EXPERIMENTAL ENDPOINT CRITERIA
1) Category I clinical signs: Mandatory euthanasia should be performed if an animal scored 3 for any clinical signs from column 3.
Separate from the condition above, the animals will be closely monitored or euthanised based on the total scoring system as given below.
Total Score Classification/Action 0-2 Good Health 3-8 Health to be monitored daily 9-12 Poor Health – Observe animals twice daily, if no improvement within 48 hours mandatory euthanasia. Seek further advice from the BRF Manager or AWO 13 and Mandatory euthanasia above
2) Category II clinical signs: Mandatory euthanasia should be performed if an animal scored 6 for any clinical sign from column 3. This includes acute ( >10%) or chronic ( >15%) weight loss unless justified and approved by the Austin AEC.
Animals with clinical signs with a single score of 4 from Category II will be closely monitored and symptoms recorded once daily. Animals with a single score of 5 require from Category II require twice daily monitoring and symptoms recorded in the morning and afternoon. Seek advice from the BRF Manager or AWO.
If there no improvement twice daily monitoring must continue until animal improves/recovers (or euthanized), then once daily monitoring is required for a minimum of 5 days unless the animal deteriorates.
Separate from the two conditions above, the animals will be closely monitored or euthanised based on the total scoring system as given above.
V2 Feb 2015 3