EQUILIBRIUM
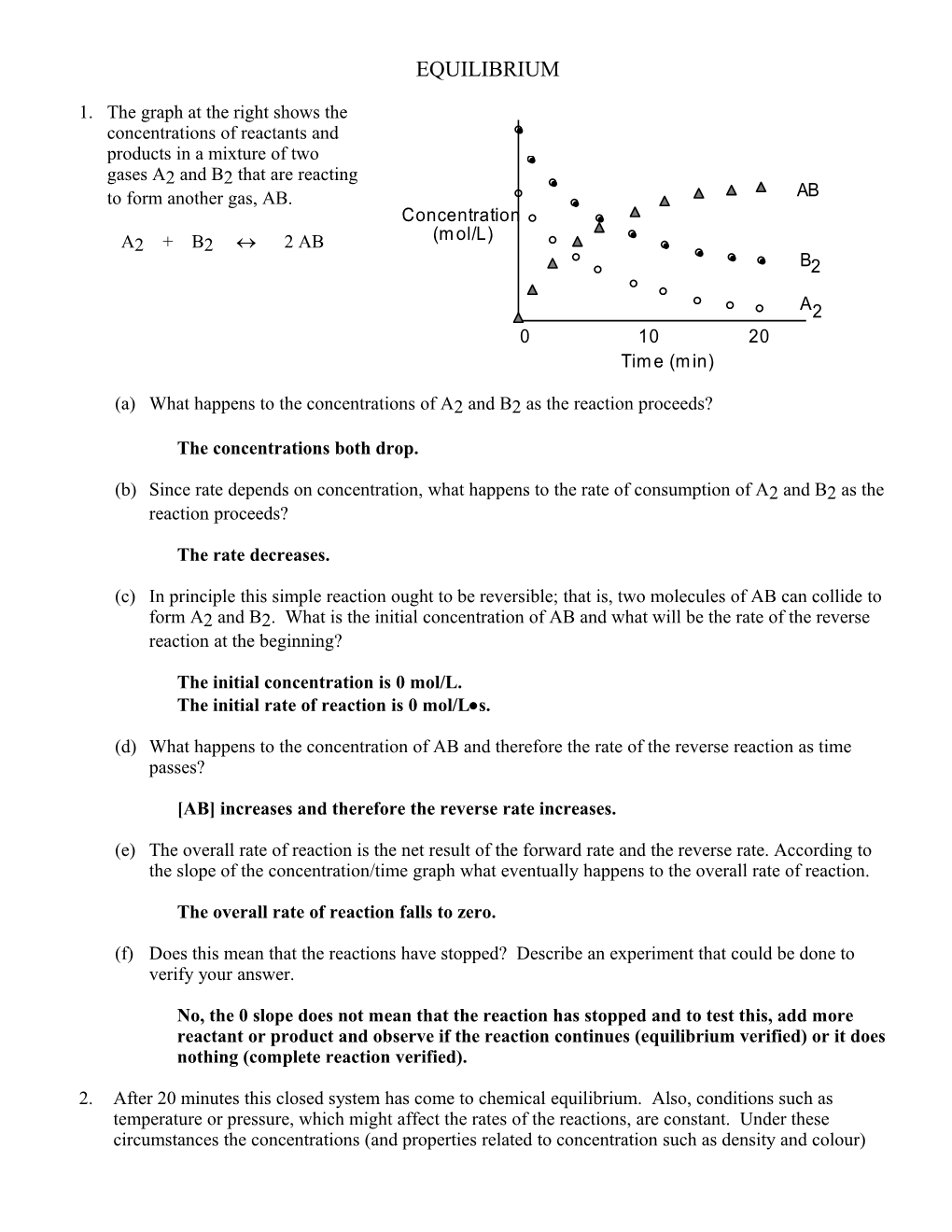
1. The graph at the right shows the concentrations of reactants and products in a mixture of two gases A2 and B2 that are reacting to form another gas, AB. AB Concentration (m ol/L) A2 + B2 2 AB B2
A2 0 10 20 Tim e (m in)
(a) What happens to the concentrations of A2 and B2 as the reaction proceeds?
The concentrations both drop.
(b) Since rate depends on concentration, what happens to the rate of consumption of A2 and B2 as the reaction proceeds?
The rate decreases.
(c) In principle this simple reaction ought to be reversible; that is, two molecules of AB can collide to form A2 and B2. What is the initial concentration of AB and what will be the rate of the reverse reaction at the beginning?
The initial concentration is 0 mol/L. The initial rate of reaction is 0 mol/Ls.
(d) What happens to the concentration of AB and therefore the rate of the reverse reaction as time passes?
[AB] increases and therefore the reverse rate increases.
(e) The overall rate of reaction is the net result of the forward rate and the reverse rate. According to the slope of the concentration/time graph what eventually happens to the overall rate of reaction.
The overall rate of reaction falls to zero.
(f) Does this mean that the reactions have stopped? Describe an experiment that could be done to verify your answer.
No, the 0 slope does not mean that the reaction has stopped and to test this, add more reactant or product and observe if the reaction continues (equilibrium verified) or it does nothing (complete reaction verified).
2. After 20 minutes this closed system has come to chemical equilibrium. Also, conditions such as temperature or pressure, which might affect the rates of the reactions, are constant. Under these circumstances the concentrations (and properties related to concentration such as density and colour) will be constant. Predict what would happen to the concentrations over the next 20 minutes if an additional 2 mol/L of B2 is suddenly injected into the reacting mixture. Explain your predictions in terms of collision theory.
The sudden increase in B2 concentration will result in additional A2 - B2 collisions which will produce additional AB. As a result there will be a decrease in A2 concentrations, a sudden increase followed by a decrease in B2 concentrations and an increase in AB concentrations. The rise in [AB] would result in increased reverse reaction slowing the [A2] and [B2] decrease until rate forward = rate reverse and the new equilibrium is reached.
3. This same reaction could be carried out in a process where A2 and B2 are added and AB is removed continually at the same rate. This would also result in constant concentrations of reactants and products.
(a) Why should this be called a steady state rather than a dynamic equilibrium?
Since the forward rate does not match the reverse rate, the constancy of concentrations is a result of removal of products and the addition of reactants rather than an equilibrium.
(b) What would be the advantages of such a process for commercial production of a substance in industry?
This process allows the continuous production of product rather than a batch mode in which the process must be started and stopped to remove product.
4. To ensure that a system is at equilibrium one must test its reversibility. If the system can be shown to change concentrations when some factor such as pressure or temperature is altered, and then return to the original concentrations when the original conditions are re-established, then it is demonstrated that the reaction is readily reversible. Otherwise, apparently constant concentrations in a system could be due to a high activation energy, and a correspondingly slow reaction rate.
(a) In a reaction similar to that above, two other substances may combine as shown in the equation
X2 (g) + Y2 (g) 2 XY(g)
When 6 moles of X2 , 4 moles of XY and 2 moles of Y2 were compressed into a one litre steel cylinder at 35°C, the concentrations were found to remain constant, and no significant amount of product was detected. What test would you do to determine whether this system is at equilibrium? If it were at equilibrium under these conditions, what would you observe?
Change the temperature and measure the new reactant and product concentrations. If the system is an equilibrium one, the concentrations will have changed. If it was at equilibrium there would be no visible change of macroscopic properties.
(b) When methane gas is mixed with air in a steel cylinder at room temperature the concentration does not change over a long period of time. What could be done to determine whether the mixture is at equilibrium?
Change the pressure or temperature. If the system is an equilibrium one, the concentrations will have changed. What do you expect to observe? Unless the temperature and/or the pressure is raised a great deal, expect no change since the activation energy for this reaction is quite high and the reaction is very exothermic. Once the rection is triggered it will be explosive as the released enthalphy sustains the reaction. Since the reverse activation energy is even larger than the forward one, little reverse reaction is expected. The reaction essentially goes to completion.
THE EQUILIBRIUM CONSTANT
1. What happens to the equilibrium constants of the reactions below as the temperature increases? Heat of reaction may be calculated from the ∆Hf° values given in appendix in the textbook.
(a) 2 NH3 N2 + 3 H2
∆H° = (1 mol(0) + 3 mol(0)) - (2 mol(-45.9 kJ/mol) = +91.8 kJ (endothermic) Ea is largest in the direction and the reaction is most affected by a temperature increase Keq which equals the ratio kf /kr increases.
(b) 2 CO(g) + O2 (g) 2 CO2 (g)
∆H° = (2 mol(-393.5 kJ/mol)) – (2 mol (-110.5 kJ/mol)) + = -566.0 kJ (exothermic) Ea is largest in the direction and the reaction is most affected by a temperature increase, Keq which equals the ratio kf /kr decreases.
2. What happens to the equilibrium constants of the following reactions as the temperature decreases?
(a) N2H4 + H2 2 NH3
∆H° = (2 mol(-45.9 kJ/mol)) - (1 mol(50.6 kJ/mol + 1 mol(0)) = -142.4 kJ (exothermic) Ea is largest in the direction and the reaction is most affected by a temperature decreaaes, Reverse of #1 - Keq increase.
(b) 16 H2S + 8 SO2 16 H2O + 3 S8
∆H° = (16 mol(-285.8 kJ/mol)) - (16 mol(-20.6kJ/mol) + 8 mol(-296.8 kJ/mol)) = -1868.8 kJ (exo), Ea is largest in the direction and the reaction is most affected by a temperature decreaaes, Reverse of #1 - Keq increase.
3. The value of Keq depends on how the chemical equation is written. Reversing the equation gives the inverse of the equilibrium constant. Changing the molar coefficients in the equation alters the powers in the equilibrium law expression. If the equilibrium constant, Keq = 40, for the reaction A2 (g) + B2 (g) 2 AB(g)
(a) What is the equilibrium constant for the reaction
2 AB(g) A2 (g) + B2 (g) 1 1 If Keq 40 then Keq' 0.025 Keq 40
(b) What is the equilibrium constant for the reaction
A2 (g) + B 2 (g) AB(g)
4. The yield of products at equilibrium is related to the magnitude of Keq. A large Keq corresponds to a relatively large amount of products at equilibrium. For which reaction below is a greater percentage of reactant converted to product; that is, which has the greater yield at equilibrium? (a) HC2H3O2 (aq) H + C 2H3O Keq = 1.8 x 10-5 (b) Fe + SCN FeSCN Keq = 300
Reaction (b) will have the greater yield
5. Only substances appearing in the equilibrium law expression will be those in solution. Solids are omitted. Write equilibrium law expressions for the following reactions:
(a) CaCO3 (s) CaO(s) + CO2 (g)
Keq = [CO2]
(b) NH3 (g) + HCl(g) NH4Cl(s)
1 Keq [NH3][HCl]
6. The equilibrium law as explained in this course applies only to dilute solutions. Since the concentration of the solvent in a dilute solution is large and nearly constant, the solvent is not usually included in the equilibrium law expression. Write equilibrium law expressions for the following reactions: (a) H2SO4 (aq) + H2O(l) HSO + H 3O 1- [HSO4 ][H3O ] Keq [H2SO4]
(b) NH3 (aq) + H2O(l) NH + OH [NH4 ][OH ] Keq [NH3]
7. When 0.40 moles of N2 is placed in a 5.0 L container with 0.20 moles of H2 it reaches an equilibrium in which there is 0.10 mol H2. The reaction equation is
N2 (g) 3 H2 (g) 2 NH3 (g) I 0.40 0.20 0 C - x - 3x 2x E 0.40 - x 0.10 2x
(a) Calculate the number of moles of H2 consumed in reaching equilibrium.
n (0.20 0.10) mol 0.10 mol H2
(b) Calculate the number of moles of N2 consumed in reaching equilibrium.
1 mol N2 nN 0.10 mol H2 x 0.033 mol 2 3 mol H2
(c) Calculate the number of moles of NH3 produced in reaching equilibrium.
2 mol NH3 nNH 0.10 mol H2 x 0.067 mol 3 3 mol H2
(d) Calculate the equilibrium concentrations of the reactants and products.
(0.40 0.033) mol [N2] 0.073 mol/L 5.0 L (0.20 0.10) mol [H2] 0.020 mol/L 5.0 L 0.067 mol [NH3] 0.013 mol/L 5.0 L
(e) Write the equilibrium law expression for this reaction.
[NH ]2 K 3 eq 3 [N2][H2] (f) Calculate the equilibrium constant for this reaction.
2 2 [NH3 ] (0.013) Keq 3 3 290 [N2 ][H2 ] (0.073)(0.020) Keq and ICE Problems Worksheet
1. Calculate the equilibrium constant, Keq, for the following reaction at 25 °C, if [NO]eq = 0.106 M, [O2]eq = 0.122 M and [NO2]eq = 0.129 M. (Answer: Keq = 12.1)
2 [NO2 ] 2 NO (g) + O2 (g) 2 NO2 (g) ; Keq 2 [NO] [O2 ] E 0.106 0.122 0.129
[0.129]2 K 12.1 eq [0.106]2[0.122]
2. Given the balanced equation and the value for Keq from #1, calculate new value of Keq for the following: (Answer: a. Keq = 1.87, b. Keq = 0.0826, Keq = 0.287)
a. 1/2 NO (g) + 1/4 O2 (g) 1/2 NO2 (g)
1/ 4 4 Multiply original reaction by 1/4, then K'eq Keq 12.1 1.87
b. 2 NO2 (g) 2 NO (g) + O2 (g)
1 -1 Multiply original reaction by -1 then K'eq Keq (12.1) 0.0826
c. NO2 (g) NO (g) + 1/2 O2 (g)
1/ 2 1 Multiply original reaction by – 1/2 then K' K 0.287 eq eq 12.1
3. Find the equilibrium constant, Keq, for the following equilibrium. The initial concentrations of AB and A2D are 0.30 M before they are mixed and when equilibrium is reached, the equilibrium concentration of A2D is 0.20 M. Be sure to show an ICE table for your calculation. (Answer: Keq = 0.80)
2 AB (g) + C2D (s) A2D (g) + 2 CB (s) I 0.30 n/a 0.30 n/a C +2x -x E 0.30 + 2x 0.30 –x = 0.20
[A2D] 0.30 - x 0.20 x 0.10 [AB] 0.30 2(0.10) 0.50 [A D] 0.20 K 2 0.80 eq [AB]2 0.502 -5 4. If 0.50 mol of NO2 is placed in a 2.0L flask to create NO and O2, calculate [ ]eq if Keq = 1.2 x 10 . 2 [NO] [O2 ] 2 NO2 2 NO O2 ; Keq = 2 [NO2 ] I 0.25 0 0 = 1.2 x 10-5
C - 2 x + 2 x + x Q 0 Keq E 0.25 - 2 x + 2 x + x 100 Rule applies 0.25 0.25 - 2 x 0.25 = 20 833 100 1.2 x 10-5
5. For the system, if we start with 0.100mol/L of CO2 and H2, what are the concentrations of the reactants and products at equilibrium given that Keq = 0.64 at 900K?
[CO][H2O] CO2 + H2 CO H2O Keq 0.64 [CO2][H2] I 0.100 0.100 0 0 Q 0 Keq C - x - x + x + x 100 Rule doesn't apply E 0.100 - x 0.100 - x + x + x
(x)(x) x 2 Keq 0.64 then 2 0.64 (0.100 - x)(0.100 - x) (0.100 - x) x 0.80 0.100 - x x 0.080 - 0.80x 1.80x 0.080 and x 0.044
[CO]eq [H2O]eq 0.044 M and [CO2 ]eq [H2 ]eq 0.100 - 0.044 0.056 M (0.044)2 Check : Q 0.62 (0.056)2 6. For the system, if we start with 0.010 mol/L of H2 and I2 and 0.096 mol/L of HI, what are their concentrations at equilibrium given that Keq = 0.016?
[H ][I ] 2 HI H I K 2 2 0.016 2 2 eq [HI]2 0.0102 I 0.096 0.010 0.010 Q 0.010 Keq 0.0962 C - 2x x x 100 Rule 0.010 E 0.096 - 2x 0.010 x 0.010 x 0.625 doesn't apply 0.016 (0.010 x)(0.010 x) (0.010 x)2 K 0.016 then 0.016 eq (0.096 - 2x)2 (0.096 - 2x)2 0.010 x 0.126 0.096 - 2x 0.010 x 0.012 - 0.252x 1.252x 0.002 x 0.0016
[HI]eq 0.096 - 2(0.0016) 0.093 M and [H2 ]eq [I2 ]eq 0.010 0.0016 0.012 M (0.012)2 Check : Q 0.017 (0.093)2
7. At 650°C, the reaction below has a Keq value of 0.771. If 2.00 mol of both hydrogen and carbon dioxide are placed in a 4.00 L container and allowed to react, what will be the equilibrium concentrations of all four gases?
[CO][H2O] H2 CO2 CO H2O K eq 0.771 [CO2 ][H2 ] I 0.500 0.500 0 0 Q 0 Keq C - x - x x x 100 rule E 0.500 - x 0.500 - x x x doesn't apply (x)(x) x 2 K 0.771 then 0.771 eq (0.500 - x)(0.500 - x) (0.500 - x)2 x 0.878 0.500 - x x 0.439 - 0.878x then x 0.234
[CO]eq 0.234M , [H2O]eq 0.234 M and [H2 ]eq [CO2 ]eq 0.500 - 0.234 0.266 M (0.234)2 Check : Q 0.774 (0.266)2 8. Carbonyl bromide, COBr2, can be formed by reacting CO with Br2. A mixture of 0.400 mol CO, 0.300 mol Br2, and 0.0200 mol COBr2 is sealed in a 5.00L flask. Calculate equilibrium concentrations for all gases. [COBr2 ] CO Br2 COBr2 Keq 0.680 [CO][Br2 ] I 0.0800 0.0600 0.00400 Q 0.83 Keq C x x - x 100 Rule - doesn't apply E 0.0800 x 0.0600 - x 0.00400 - x 0.00400 - x K 0.680 eq (0.0800 x)(0.0600 x) 0.00400 - x 0.680 (0.0048 0.14x x 2 ) 0.00400 - x 0.0033 0.095x 0.680x 2 0.680x 2 1.095x - 0.0007 0 a 0.680 b 1.095 c - 0.0007 -1.095 1.20 x 1.36 -1.095 1.096 x 1.36 x 0.0007 or x -1.611 (no negatives)
[CO]eq 0.0807 M, [Br2 ]eq 0.0607 M and [COBr2 ]eq 0.00400 0.0007 0.0033M (0.0033) Check : Q 0.674 (0.0807)(0.0607) DETERMINATION OF Keq
OBJECTIVE: Determine the equilibrium constant for the reaction Fe + SCN FeSCN
INTRODUCTION: Iron(III) ion reacts with thiocyanate ion to produce a coloured complex ion.
Fe + SCN FeSCN
The intensity of the colour of the solution depends on two things, the concentration of the coloured species and the path length through which light travels in the solution. For example, light traveling through a 3 cm depth of a 0.1 mol/L solution of this ion will appear to have the same colour intensity as light traveling through a 1 cm depth of a 0.3 mol/L solution. Therefore, if the depth of two solutions is adjusted until they appear to have the same colour intensity, the ratio of their concentrations will be inversely proportional to the ratio of their depths: = If the concentration of one solution is known then concentration of the other may be calculated. The equilibrium concentrations may be used to calculate the value of the equilibrium constant for this reaction. Then predictions may be made about the equilibrium concentrations in other solutions.
To prepare a solution with a known concentration of the coloured complex, a dilute solution of thiocyanate ion is reacted with a concentrated solution of iron (III) ion. It may be assumed that the large excess of iron (III) ion causes most of the thiocyanate ion to be converted to the coloured complex. Therefore, for this mixture the equilibrium concentration of the coloured complex will be equal to the initial concentration of the thiocyanate ion.
PROCEDURE: (a) Line up five clean test tubes all of the same diameter, and label them. Add 5.0 mL of 0.002 mol/L potassium thiocyanate solution to each of these five test tubes. To test tube (1) add 5.0 mL of 0.2 mol/L iron (III) nitrate solution. This tube will be used as the standard.
(b) Measure 10.0 mL of 0.2 mol/L iron (III) nitrate solution in a graduated cylinder, and fill to the 25.0 mL mark with distilled water. Pour the solution into a clean dry beaker to mix it. Measure 5.0 mL of this solution and pour it into test tube 2. Save the remainder of this solution for Part (c).
(c) Pour 10.0 mL of the diluted iron (III) nitrate solution from the beaker into your graduate. Discard the remainder. Again fill the graduate to the 25.0 mL mark with distilled water, and pour the solution into a clean dry beaker to mix. Pour 5.0 mL of this solution into test tube 3. Continue dilution in this manner until you have 5.0 mL of successively more dilute solution in each test tube.
(d) Now compare the solutions in each of the test tubes with the standard tube (1) in order to determine the concentration of the coloured complex ion. Wrap a strip of paper around test tubes (1) and (2) to exclude light from the side. Look vertically down through the solutions toward a diffused light source. If the colour intensities appear the same, measure the depth of each solution to the nearest millimetre and record this. If the colour intensities do not appear the same, remove some of the solution form the standard tube with a dropper until the colour intensities are the same. Put the portion you removed into a clean dry beaker, since you may have to use some of this solution later. The matching may be accomplished by removing more standard than seems necessary and then replacing part of it drop by drop. When the colour intensities are the same in each test tube, measure the depth of both solutions to the nearest millimetre. Repeat the procedure comparing test tubes (1) and (3), (1) and (4), (1) and (5). CALCULATIONS
1. Remember that the solution in test tube (1) was taken as the standard. The equilibrium concentration of coloured complex in this solution is very nearly equal to the concentration of thiocyanate in the mixture before the reaction began. However, a calculation is necessary since the 0.002 mol/L solution of thiocyanate used was diluted by the addition of the other solution even before the reaction occurred. The initial concentration of thiocyanate in the mixture is found by multiplying its concentration before mixing (0.002) by the dilution factor, (initial volume/final volume), which in this case is (5/10). Therefore for the standard solution (1), the equilibrium concentration of the coloured complex was
2+ [FeSCN ]eq = 0.002 x (5/10) mol/L.
2. The initial concentration of thiocyanate ion in each test tube was
-1 [SCN ]in = 0.002 x (5/10) mol/L.
3. The initial concentration of the iron (III) ion was different in each test tube since this solution was diluted repeatedly by a factor of (10/25) in preparing the other solutions. In addition, the iron (III) ion was further diluted by mixing with the thiocyanate solution.
3+ n-1 [Fe ]in = 0.2 x (10/25) x (5/10) mol/L where n is the test tube number.
4. The equilibrium concentration of the coloured complex in each test tube is equal to the assumed concentration in (1) multiplied by the ratio of depths which you measured in your experiment.
5. The amount of thiocyanate ion consumed is equal to the amount of coloured ion produced. Therefore to get the equilibrium concentration of thiocyanate ion, subtract the equilibrium concentration of the coloured product from the initial concentration of the thiocyanate ion.
6. Similarly, subtract the equilibrium concentration of the coloured product from the initial concentration of ferric ion to get the equilibrium concentration of ferric ion.
7. Write the equilibrium expression for this reaction.
8. Calculate the values for the equilibrium constant from the data for the second through fifth test tubes.
9. Summarize your calculations in the data table below. 1 2 3 4 5 Initial [SCN-1] 0.00100 0.00100 0.00100 0.00100 0.00100 Initial [Fe3+] 0.100 0.0400 0.0160 0.00640 0.00256
Depth of Standard ( D1) 5.20 4.60 3.90 2.70 1.50
Depth of Sample (D2) 5.20 5.20 5.20 5.20 5.20
Depth Ratio (D1/D2) N/A 0.8846 0.75 0.5192 0.2885 Equilibrium [FeSCN2+] 0.001 8.85x10-4 7.50x10-4 5.19x10-4 2.88x10-4 Equilibrium [SCN-1] ~0 1.15x10-4 2.50x10-4 4.81x10-4 7.12x10-4 Equilibrium [Fe3+] N/A 3.91x10-2 1.53x10-2 5.92x1--3 2.27x10-3
Keq N/A 197 196 182 178
CONCLUSION: What is the average Ke value for the mixtures in test tubes 2 to 5? 197 196 182 178 188 4 LE CHATELIER'S PRINCIPLE
1. The graph at the right shows the affect of adding more reactant, B , 2 6 [A ] (at time = 20 minutes) to a system 2 initially at equilibrium with [AB] concentrations of 6 mol/L, 2 mol/L 4 and 4 mol/L respectively for A2, B2 Concentration [B2 ] and AB. (mol/L) 2
0 15 20 25 30 35 Time The reaction is (min) A2 + B2 2 AB Use collision theory to explain the change in concentrations.
The addition of B2 results in an increased number of collisions between the reactants which causes a decrease in the concentrations of A2 and B2 and an increase in the concentration of the product AB. As. the reactants are consumed the forward reaction rate decreases and as the product is produced, the reverse reaction rate increases until the forward and reverse rates become equal and equilibrium is reestablished.
2. If pressure on the system is increased, the equilibrium will shift to reduce the number of moles of gas in the mixture, thus lowering the pressure again. What would be the affect on the yield of products of increasing the pressure in each of the following systems at equilibrium?
(a) 3 H2 (g) + N2 (g) 2 NH 3 (g)
Increased pressure results in a greater increase in reactant particle collisions than products and a greater increase in the forward reaction than the reverse and requires a reduction in gaseous moles which is the product side therefore increased products
(b) 2 NaCl(s) + H2SO4 (l) 2 HCl(g) + Na2SO4 (s)
Increased pressure results in an increase in product collisions and an increase in the reverse reaction and requires a reduction in gaseous moles which is the reactant side therefore decreased products
(c) SO3 (g) + CaCO3 (s) CaSO4 (s) + CO2 (g)
Since the gaseous moles are the same for reactants and products therefore nothing happens 3. If the temperature is raised, the equilibrium will shift in the endothermic direction to consume some of the added heat. What would be the affect of raising the temperature on the concentrations of products in each of the following systems at equilibrium? Explain using Collision Theory.
(a) HCl + NH3 NH4Cl + heat
To absorb the increased heat the reverse reaction must proceed therefore product concentrations decrease, OR since exothermic, activation energy in the reverse is larger and therefore the reverse reaction increases more than the forward and product concentrations decrease. (b) 1/2 N2 + 3/2 H2 NH3 ∆H = -46.2 kJ/mol NH3
To absorb the increased heat the reverse reaction must proceed therefore product concentrations decrease, OR since exothermic, activation energy in the reverse is larger and therefore the reverse reaction increases more than the forward and product concentrations decrease.
(c) C2H4 (g) + heat C2H2 (g) + H2 (g)
To absorb the increased heat the forward reaction must proceed therefore product concentrations increase, OR since endothermic, activation energy in the forward is larger and therefore the forward reaction increases more than the reverse and product concentrations increase.
4. Of course, lowering the concentration, pressure or temperature will have the opposite affect to the changes described above. Each question below refers to the reaction: N2O4 (g) + heat 2 NO2 (g)
(a) What is the affect on the yield of products of decreasing the pressure? Increase
(b) What would be the affect of decreasing the temperature? Decrease
(c) What would be the affect of removing the NO2 as it is formed? Increase
5. H2(g) + Cl2(g) 2 HCl(g)
What direction will the equilibrium shift when the partial pressure of hydrogen is increased? It will shift to the right to decrease the pressure of hydrogen.
6. 3 H2(g) + N2(g) 2 NH3(g)
Given that this reaction is exothermic, what direction will the equilibrium shift when the temperature of the reaction is decreased? It will shift to the right so that the heat that’s being removed will be replaced.
7. 2 NO2(g) N2O4(g)
If a large quantity of argon is added to the container in what direction will the equilibrium shift? It won’t shift, because the partial pressures of each gas will be the same. 8. NH4OH(aq) NH3(g) + H2O(l)
In what direction will the equilibrium shift if ammonia is removed from the container as soon as it is produced? It will shift to the right in an effort to increase the quantity of ammonia present.
9. 2 BH3(g) B2H6(g)
If this equilibrium is taking place in a piston with a volume of 1 L and I compress it so the final volume is 0.5 L, in what direction will the equilibrium shift? It will shift to the right so the volume of the gases in the equilibrium will also be decreased. PART 1 Equilibrium Involving Thymol Blue 2- + 1- Tb (blue) + H3O ⇄ HTb (yellow) + H2O 1- + HTb (yellow) + H3O ⇄ H2Tb (red) + H2O stress [ ] change [ ] shift direction [ ] visible change
Starting blue [H3O+] [Tb2-] [HTb 1-] yellow HCl 1st
HCl 2nd [H3O+] [HTb2-] [H2Tb ] red
NaOH 1st [H3O+] [H2Tb] [HTb 1-] yellow
NaOH 2nd [H3O+] [HTb 1-] [Tb2-] blue
PART 2 Equilibrium involving Thiocyanatoiron (III) Ion 3+ - 2+ Fe (aq) + SCN (aq) ⇄ FeSCN (aq) stress [ ] change [ ] shift direction [ ] visible change
Fe(NO3) Dark [Fe3+] [SCN-] [FeSCN2+] 3 orange/red Dark KSCN - 3+ 2+ [SCN ] [Fe ] [FeSCN ] orange/red
[Fe3+] as NaOH Fe(OH)3 solid [FeSCN2+] [Fe3+] yellow forms
HCl [H2O] - dilution Nothing no change Nothing lighter
PART 3 Equilibrium Involving Copper Complexes 2+ 2+ Cu(H2O)4 (aq) + 4 NH3 (aq) ⇄ Cu(NH3)4 (aq) + 4 H2O + - Also NH3 (aq) + H2O (l) ⇄ NH4 (aq) + OH (aq) stress [ ] change [ ] shift [ ] visible change direction
[Cu(H O) 2+] NH3 [Cu(H2O)42+] as 2 4 Light blue with [Cu(NH ) 2+] 1st Cu(OH) solid forms 3 4 precipitate 2 or [NH3]
NH3 Darker blue [NH ] [Cu(H O) 2+] [Cu(NH ) 2+] 2nd 3 2 4 3 4 soultion [NH3] & Light blue HCl [H3O+], then [OH-] [Cu(H2O)42+] [Cu(NH3)42+] solution Equilibrium Constant, Keq
1. Once a system has reached equilibrium, are the following true or false? a. The reaction is finished, no more products are forming. ____false______b. The concentrations of the reactants and the products are equal. ____false______c. The concentrations are no longer changing. ____false__ d. The reaction is not over, but will continue forever if isolated. ____true____ e. The speed at which products are made equals the speed at which reactants form. ___true___
2. What is equal at equilibrium? ______rate forward = rate reverse______
3. What general information can be gathered by observing the magnitude of the equilibrium constant?
Whether the reactants or products are favoured.
4. Write the expression for Keq for the reaction: 2 NO (g) + Cl2 (g) 2 NOCl (g) [NOCl]2 K eq 2 [NO] [Cl2 ]
5. Write the Keq for: 2 K3PO4 (aq) + 3 Ca(NO3)2 (aq) 6 KNO3 (aq) + Ca3(PO4)2 (s) [KNO ]6 K 3 eq 2 3 [K3PO4 ] [Ca(NO3)2 ]
6. Write the expression for Keq for the reaction : H2 (g) + Br2 (l) 2 HBr (g) [HBr]2 Keq [H2]
7. Write the expression for Keq for the reaction: CO2 (g) + CaO (s) CaCO3 (s) 1 Keq [CO2 ]
8. For the reaction: SiH4 (g) + 2 O2 (g) SiO2 (g) + 2 H2O (l) a) Write the equilibrium expression for the forward reaction: [SiO ] K 2 eq 2 [SiH4][O2]
b) Write the equilibrium expression for the reverse reaction: 2 [SiH4][O2] 1 Keq ' [SiO2] Keq
c) What is the equilibrium constant in the forward direction if [SiH4] = 0.45M; [O2] = 0.25M; and [SiO2] = 0.15M at equilibrium? [SiO ] (0.15) K 2 5.3 eq 2 2 [SiH4][O2 ] (0.45)(0.25) d) What is the equilibrium constant in the reverse reaction? 1 Keq 0.19 5.3
e) If [SiH4] = 0.34M; [O2] = 0.22M and [SiO2] = 0.35M, what would be the reaction quotient (Q) in the forward direction and which direction will the reaction go? (0.35)(0.20)2 Keq 0.85 (0.34)(0.22)2 Q = 0.85 > Keq = 0.53 then the reaction will go towards the products
Calculating Keq, Q, ICE tables
9. Calculate the equilibrium constant for this reaction: 2 PO2Br (aq) 2 PO2 (aq) + Br2 (aq) Given: [PO2Br] = 0.0255M, [PO2] = 0.155M, and [Br2] = 0.00351M at equilibrium.
[PO ]2[Br ] 2 PO Br 2 PO Br K 2 2 2 2 2 eq 2 [PO2Br] (0.155)2 (0.00351) Keq 0.130 (0.0255)2
10. For the combination reaction: H2 (g) + F2 (g) 2 HF (g), calculate all three equilibrium concentrations when [H2]i = [I2] i = 0.200 M and Keq = 64.0. [HF]2 H2 F2 2 HF Keq 64.0 [H2 ][F2 ] I 0.200 0.200 0 Q 0 Keq C - x - x 2x 100 Rule - doesn't apply 0.200 E 0.200 - x 0.200 - x 2x 0.003125 100 64.0 (2x)2 K 64.0 perfect square eq (0.200 - x)(0.200 - x) (2x)2 64.0 (0.200 - x)(0.200 - x)
2x 8.00 0.200 - x 2x 1.60 - 8.00x 10.00x 1.60 1.60 x 0.160 10.00 [H2 ]eq [F2 ]eq 0.200 - 0.160 0.040 M and [HF]eq 2(0.160) 0.320 M (0.320)2 Check : Q 64.0 (0.040)2
11. For the decomposition reaction, COCl2 (g) CO (g) + Cl2 (g), calculate all three equilibrium concentrations when Keq = 0.680 with [CO]i = 0.500 and [Cl2]i = 1.00 M. [CO][Cl2] COCl2 CO Cl2 Keq 0.680 [COCl2] I 0 0.500 1.00 Q undef Keq C x - x - x 100 Rule - doesn't apply 0.500 E x 0.500 - x 1.00 - x 0.735 100 0.680 (0.500 - x)(1.00 - x) Keq 0.680 x 0.500 - 1.50x x2 0.680 x
x2 - 2.18x 0.500 0 a 1 b - 2.18 c 0.500 -(-2.18) 4.7524 - 2 x 2 2.18 1.66 x 2 x 1.92 (too big) or x 0.260
[COCl2]eq 0.260M , [CO]eq 0.500 - 0.260 0.240 M and [Cl2]eq 1.00 - 0.260 0.740 M (0.240)(0.740) Check : Q 0.683 (0.260)
12. We place 0.0640 mol N2O4 (g) in a 4.00 L flask at 200 K. After reaching equilibrium, the concentration
of NO2(g) is 0.00300 M. What is Keq for the reaction N2O4(g) 2 NO2(g)? 2 [NO2 ] N2O4 2 NO2 Keq [N2O4 ] I 0.0160 0 C - x 2x E 0.0160 2x
but [NO2 ]eq 0.00300 M 2x 0.00300 x 0.00150 (0.00300)2 K 6.21 x 10-4 eq (0.0160 - 0.00150) 13. Carbonyl bromide decomposes to carbon monoxide and bromine: COBr2(g) CO(g) + Br2(g) Keq is -4 o 1.90 x 10 at 73 C. If an initial concentration of 0.300 M COBr2 is allowed to equilibrate, what are the equilibrium concentrations of COBr2, CO, and Br2?
[CO][Br2 ] -4 COBr2 CO Br2 Keq 1.90 x 10 [COBr2 ] I 0.300 0 0 Q 0 Keq C - x x x 100 Rule - applies 0.300 E 0.300 - x x x 1579 100 1.90 x 10-4 0.300 - x 0.300 (x)(x) K 1.90 x 10-4 eq 0.300 x2 5.70 x 10-5 x 7.55 x 10 -3
[COBr2 ]eq 0.300 - 0.00755 0.292 M , [CO]eq [Cl2 ]eq 0.00755 M (0.00755)2 Check : Q 1.93 x 10-4 0.292
14. PCl5 decomposes into PCl3 and Cl2 gas. What is the initial concentration of PCl5 if at equilibrium the concentration of chlorine gas is 0.500M? Given: Keq = 10.00 (Hint: ICE table) [PCl3][Cl2 ] PCl5 PCl3 Cl2 Keq 10.00 [PCl5 ] I y 0 0 C - x x x E y - x x x
and [Cl2 ]eq 0.500M x (0.500)(0.500) K 10.00 eq y - 0.500 0.25 10.00y - 5.00 10.00y 5.25 y 0.525
[PCl5]i 0.525 M and [PCl5]eq 0.525 - 0.500 0.025 M (0.500)(0.500) Check : Q 10.00 0.025
Le Chatelier’s Principle
15. Consider this endothermic reaction: 3 O2(g) 2 O3(g). To shift this reaction to the reactants:
a) You could ______the pressure. (decrease) b) You could ______the volume. (increase) c) You could ______oxygen gas. (remove) d) You could ______the temperature. (decrease) e) Which of the following, if increasing, will change the value of the equilibrium constant? Temperature
16. Consider this reaction: 2 SO2(g) + O2(g) 2 SO3(g). To shift this reaction towards the products:
a) You could ______the pressure. (increase) b) You could ______the volume. (decrease) c) You could ______oxygen gas. (add)
17. For each system described below, indicate in which direction the equilibrium will shift when each stress is added or removed.
a) N2 (g) + 3 H2 (g) 2 NH3 (g): more H2 is added to reaction at equilibrium. ()
b) For the same reaction, some NH3 is removed from the reaction at equilibrium. ()
c) 2 SO2 (g) + O2 (g) 2 SO3 (g) + heat: heat is added. () d) Using the same reaction, heat is removed ()
e) PCl3 (g) + Cl2 (g) PCl5 (g): volume is reduced by half. () f) Using the same system as above, a catalyst is added to the system. (same)
g) H2 (g) + Cl2 (g) 2 HCl (g): volume is doubled. (same) h) Using the same system as above, some neon is added to the system. (same)
18. Explain how the following changes in reaction conditions will affect the position of the equilibrium: A (g) + B (aq) C (s) ΔHrxn= -453 kJ/mol a) The pressure of A in the reaction chamber is increased. () b) The temperature of the reaction is increased by 20 0C. () c) A catalyst is added to the system. (same) d) More of compound B is steadily added to the reaction chamber. () e) More of compound C is steadily added to the reaction chamber. () f) Argon gas is added to the reaction chamber, doubling the pressure. (same) Ksp PROBLEM SET #1
1. Write the solubility expression for the following: (a) Zn(OH)2 (b) Ca3(PO4)2 (c) Fe2(SO4)3 2 - 2 - 2 (a) Zn(OH)2 (s) Zn (aq) 2 OH (aq) ; Ksp [Zn ][OH ] 2. 2 3- 2 3 3- 2 (b) Ca3(PO4)2 (s) 3 Ca (aq) 2 PO4 (aq) ; Ksp [Ca ] [PO4 ] 2 2- 2 2 2- 3 (c) Fe2(SO4)3 (s) 2 Fe (aq) 3 SO4 (aq) ; Ksp [Ca ] [SO4 ] -12 -3 Calculate the solubility of Mg(OH)2 in g/L given that Ksp = 5.6 x 10 . (6.5 x 10 g/L).
2 - 2 - 2 Mg(OH)2 Mg (aq) 2 OH (aq) ; Ksp [Mg ][OH ] ICE x 2x 5.6 x 1012 2 - 2 12 2 12 3 12 Ksp [Mg ][OH ] 5.6 x 10 ; (x)(2x) 5.6 x 10 ; 4x 5.6 x 10 x 1.12 x 104 [Mg2 ] molar solubility 4 1.12 x 10 mol Mg(OH)2 58.33 g 3 then, solubility of Mg(OH)2 x 6.5 x 10 g/L L mol Mg(OH)2
-3 -10 3. The solubility of SrF2 is 7.3 x 10 g/100 mL. Calculate Ksp. (7.8 x 10 )
2 - 2 - 2 SrF2 Sr (aq) 2 F (aq) ; Ksp [Sr ][F ] ICE x 2x 7.3 x 10-3 g 1 mol SrF 100 mL molar solubility x x 2 x 5.81 x 104 mol/L 100 mL 125.62 g 0.1L then, Ksp [Sr 2 ][F- ]2 (x)(2x)2 4x3 4(5.81 x 104 )3 7.8 x 1010
-3 -7 4. The solubility of Cu(IO3)2 is 3.245 x 10 mol/L. Calculate Ksp. (1.367 x 10 )
2 - 2 - 2 Cu(IO3)2 Cu (aq) 2 IO3 (aq) ; Ksp [Cu ][IO3 ] ICE x 2x 2 - 2 3 3 2 7 Ksp [Cu ][IO3 ] (3.245 x 10 )(2 x 3.245 x 10 ) 1.367 x 10
-9 5. The solubility of PbI2 is 0.058 g/100mL. Calculate the Ksp for PbI2. (8.0 x 10 ) 2 - 2 - 2 PbI2 Pb (aq) 2 I (aq) ; Ksp [Pb ][I ] ICE x 2x 0.058 g 1 mol PbI 100mL molar solubility x x 2 x 1.26 x 103 mol/L 100 mL 461.00 g 0.1L then, Ksp [Pb2 ][I- ]2 (x)(2x)2 4x3 4(1.26 x 103 )3 8.0 x 109
2+ -9 6. Calculate the molar concentration of Ba ion in a saturated soln of Ba(IO3)2, if the Ksp = 1.15 x 10 . (6.6 x 10-4 mol/L)
2 - 2 - 2 Ba(IO3 )2 Ba (aq) 2 IO3 (aq) ; Ksp [Ba ][IO3 ] ICE x 2x 1.15 x 109 2 - 2 9 2 9 3 9 Ksp [Ba ][IO3 ] 1.15 x 10 ; (x)(2x) 1.15 x 10 ; 4x 1.15 x 10 x 6.6 x 104 [Ba2 ]
-4 -14 7. The solubility of Cd(OH)2 is 2.01 x 10 g/100mL. Calculate Ksp. (1.04 x 10 )
2 - 2 - 2 Cd(OH)2 Cd (aq) 2 OH (aq) ; Ksp [Cd ][OH ] ICE x 2x 2.01 x 10-4 g 1 mol Cd(OH) 100mL molar solubility x x 2 x 1.373 x 105 mol/L 100 mL 146.43 g 0.1L then, Ksp [Pb2 ][I- ]2 (x)(2x)2 4x3 4(1.37 x 105 )3 1.04 x 1014
8. The Ksp for Silver Phosphate is 2.8 x 10-18. Calculate the solubility in g/100 mL. (7.49 x 10-4 g/100 mL)
3- 3 3- Ag3PO4 3 Ag (aq) PO4 (aq) ; Ksp [Ag ] [PO4 ] ICE 3x x 2.8 x 1018 3 3- 18 3 18 4 18 Ksp [Ag ] [PO4 ] 2.8 x 10 ; (3x) (x) 2.8 x 10 ; 27x 2.8 x 10 5 3- x 1.79 x 10 [PO4 ] molar solubility 5 1.79 x 10 mol Ag3PO4 418.58 g 3 then, solubility of Ag3PO4 x 7.49 x 10 g/L L mol Ag3PO4 7.49 x 104 g/100 mL
-4 -11 9. The solubility of Mg(OH)2 is 9.0 x 10 g/100 mL at 18°C. Calculate Ksp. (1.5 x 10 ) 2 - 2 - 2 Mg(OH)2 Mg (aq) 2 OH (aq) ; Ksp [Mg ][OH ] ICE x 2x 9.0 x 10-4 g 1 mol Mg(OH) 100mL molar solubility x x 2 x 1.54 x 104 mol/L 100 mL 58.33 g 0.1L then, Ksp [Pb2 ][I- ]2 (x)(2x)2 4x3 4(1.54 x 104 )3 1.5 x 1011 Ksp PROBLEM SET #2
-5 1. If 0.010 mg of NaCl is added to 200. mL of a 2.0 x 10 M AgNO3 will a precipitate form? Ksp AgCl = 1.8 x 10-10. (Q = 1.7 x 10-11)
NaCl Na Cl- 1mol NaCl 1 M 58.44 g/mol 1.0 x 10-5 g x x 58.44 g 0.200 L 8.56 x 10-7 mol/L - AgNO3 Ag NO3 2.0 x 10-5 - - AgCl Ag (aq) Cl (aq) ; Ksp [Ag ][Cl ] 2.0 x 10-5 8.56 x 10-7 1.8 x 10-10 Q (2.0 x 10-5)(8.56 x 10-7) 1.7 x 10-11 Q Ksp no ppte will form
2. If one gram of AgNO3 is added to 50. mL of a 0.050 M HC2H3O2 which is soluble, will a precipitate -3 -3 form? Ksp for AgC2H3O2 is 2.0 x 10 . (Q = 5.9 x 10 )
- HC2H3O2 H C2H3O2 0.050 mol/L - AgNO3 Ag NO3 1mol AgNO 1 M 169.88 g/mol 1.0 g x 3 x 169.88 g 0.050 L 0.118 mol/L - - AgC2H3O2 Ag (aq) C2H3O2 (aq) ; Ksp [Ag ][C2H3O2 ] 0.118 0.050 2.0 x 10-3 Q (0.118)(0.050) 5.9 x 10-3 Q Ksp ppte will form 3. In each of the following cases, show whether a precipitate will form under the given conditions: (a) 10. mL of 0.10 M AgNO3 is added to 600. mL of a 0.010 M Na2SO4 solution. Ksp for Ag2SO4 is 1.5 x 10-5. (Q = 2.65 x 10-8)
2- Na2SO4 2 Na SO4 0.010 mol n x 0.600 L L 6.00 x 10-3 mol - AgNO3 Ag NO3 0.10 mol n x 0.0.010 L L 1.00 x 10-3 mol 2- 2 2- Ag2SO4 2 Ag (aq) SO4 (aq) ; Ksp [Ag ] [SO4 ] 1.00 x 10-3mol 6.00 x 10-3mol 1.5 x 10-5 0.610 L 0.610 L 1.64 x 10-3 9.84 x 10-3 Q (1.64 x 10-3)2(9.84 x 10-3) 2.65 x 10-8 Q Ksp no ppte will form
-5 -6 (b) 1.0 g of Pb(NO3)2 is put in 100. mL of 0.010 M HCl. Ksp (PbCl2) = 1.2 x 10 . (Q = 3.0 x 10 )
2 - Pb(NO3)2 Pb 2 NO3 1mol Pb(NO ) 1 M 331.22 g/mol 1.0 g x 3 2 x 331.22 g 0.100 L 3.02 x 10-2 mol/L HCl H Cl- 0.010 mol/L 2 - 2 - 2 PbCl2 Pb (aq) 2 Cl (aq) ; Ksp [Pb ][Cl ] 3.02 x 10-2 0.010 1.2 x 10-5 Q (3.02 x 10-2)(0.010) 3.0 x 10-6 Q Ksp no ppte will form (c) 1.0 milligram of CaCl2 and 1.0 milligram of Na2C2O4 are added to 1.0 litre of water. Ksp for -9 -11 CaC2O4 is 2.0 x 10 . (Q = 6.7 x 10 )
2 - CaCl2 Ca 2 Cl 1mol CaCl 1 M 110.98 g/mol 1.0 x 10-3 g x 2 x 110.98 g 1.0 L 9.01 x 10-6 mol/L 2- Na2C2O4 2 Na C2O4 1mol CaCl 1 M 134.00 g/mol 1.0 x 10-3 g x 2 x 134.00 g 1.0 L 7.46 x 10-6 mol/L 2- 2- CaC2O4 Ca (aq) C2O4 (aq) ; Ksp [Ca ][C2O4 ] 9.01 x 10-6 7.46 x 10-6 2.0 x 10-9 Q (9.01 x 10-6)(7.46 x 10-6) 6.7 x 10-11 Q Ksp no ppte will form
-9 4. Given that the Ksp for CaC2O4 is 2.0 x 10 , calculate how many grams of CaC2O4 will dissolve in 1.0 litre of: -3 -6 (a) water (m = 5.7 x 10 g) (b) 0.10 M Na2C2O4 (m = 2.6 x 10 g) 2 2- 2 2- 9 (a) CaC2O4 Ca C2O4 ; Ksp [Ca ][C2O4 ] 2.0 x 10 ICE x x (x)(x) 2.0 x 109 x 4.47 x 10-5 molar solubility 4.47 x 10-5 mol CaC O 128.1 g m 2 4 x x 1.0 L 5.7 x 103 g CaC 2O 4 L 1 mol CaC2O4 2- (b) Na2C2O4 2 Na C2O4 0.10 2 2- 2 2- 9 CaC2O4 Ca C2O4 ; Ksp [Ca ][C2O4 ] 2.0 x 10 ICE x 0.10 (x)(0.10) 2.0 x 109 x 2.0 x 10-8 molar solubility 2.0 x 10-8 mol CaC O 128.1 g m 2 4 x x 1.0 L 2.6 x 106 g CaC 2O 4 L 1 mol CaC2O4 -5 -3 (c) 0.010 M CaCl2 (m = 2.6 x 10 g) (d) 0.10 M NaNO3 (m = 5.7 x 10 g) 2 - (c) CaCl2 Ca 2 Cl 0.010 2 2- 2 2- 9 CaC2O4 Ca C2O4 ; Ksp [Ca ][C2O4 ] 2.0 x 10 ICE 0.010 x (0.010)(x) 2.0 x 109 x 2.0 x 10-7 molar solubility 2.0 x 10-7 mol CaC O 128.10 g m 2 4 x x 1.0 L 2.6 x 105 g CaC 2O 4 L 1 mol CaC2O4 - (d) NaNO3 Na NO3 0.10 0.10 2 2- 2 2- 9 CaC2O4 Ca C2O4 ; Ksp [Ca ][C2O4 ] 2.0 x 10 ICE x x There is no common ion, so the m 5.7 x 103 g as in (a). CaC 2O 4
-9 5. Given the Ksp for PbI2 = 8.5 x 10 , calculate how many grams of PbI2 will dissolve in 250. mL of the following systems: (a) water (m = 1.5 x 10-1g) 2 - 2 - 2 9 PbI2 Pb 2 I ; Ksp [Pb ][I ] 8.5 x 10 ICE x 2x (x)(2x)2 8.5 x 109 ; 4x3 8.5 x 109 ; x 1.29 x 103 molar solubility 3 1.29 x 10 mol PbI2 461.00 g 1 mPbI x x 0.250 L 1.5 x 10 g 2 L 1 mol PbI2
-2 -3 (b) 0.010 M Pb(NO3)2 (m = 5.3 x 10 g) (c) 0.010 M CaI2 (m = 2.5 x 10 g) 2 - (b) Pb(NO3 )2 Pb 2 NO3 0.010 2 - 2 - 2 9 PbI2 Pb 2 I ; Ksp [Pb ][I ] 8.5 x 10 ICE 0.010 2x (0.010)(2x)2 8.5 x 109 ; x 4.6 x 104 molar solubility and m 5.3 x 102 g PbI2 2 - (c) CaI2 Ca 2 I 0.020 2 - 2 - 2 9 PbI2 Pb 2 I ; Ksp [Pb ][I ] 8.5 x 10 ICE x 0.020 (x)(0.020)2 8.5 x 109 ; x 2.13 x 105 molar solubility and m 2.5 x 103 g PbI2
-3 -4 6. 75 mL of a 2.0 x 10 M AgNO3 solution is mixed with 45 mL of a 1.0 x 10 M NaCl soln. Will a ppte form? Ksp AgCl = 1.8 x 10-10. (Q = 4.7 x 10-8) NaCl Na Cl- C 1.0 x 10-4 mol/L, V 0.045 L n 4.5 x 10-6mol - AgNO3 Ag NO3 C 2.0 x 10-3 mol/L, V 0.075 L n 1.5 x 10-4mol - - 10 AgCl Ag Cl ; Ksp [Ag ][Cl ] 1.8 x 10 1.5 x 10-4mol 4.5 x 10-6mol
0.120 L 0.120 L 1.25 x 10-3 3.75 x 10-5 Q (1.25 x 10-3)(3.75 x 10-5) 4.7 x 10-8 Q Ksp a ppte will form
7. How many grams of AgCl will dissolve in 100.0 mL of a 0.010 M NaCl soln. Ksp = 1.8 x 10-10. (m = 2.6 x 10-7g) NaCl Na Cl- 0.010 - - 10 AgCl Ag Cl ; Ksp [Ag ][Cl ] 1.8 x 10 ICE x 0.010 10 8 7 (x)(0.010) 1.8 x 10 ; x 1.8 x 10 molar solubility and mAgCl 2.6 x 10 g
8. 100. mL of a 0.010 M Mg(NO3)2 is mixed with 50. mL of a 0.010 M Ba(OH)2. Will a ppte form? Ksp -12 -7 Mg(OH)2 = 5.6 x 10 . (Q = 3.0 x 10 )
2 - Ba(OH)2 Ba 2 OH C 0.010 mol/L (x 2), V 0.050 L n 1.0 x 10-3 mol 2 - Mg(NO3 )2 Mg 2 NO3 C 0.010 mol/L, V 0.100 L n 1.00 x 10-3 mol 2 - 2 - 2 12 Mg(OH)2 Mg 2 OH ; Ksp [Mg ][OH ] 5.6 x 10 1.0 x 10-3 mol 1.00 x 10-3 mol
0.150 L 0.150 L 6.67 x 10-3 6.67 x 10-3 Q (6.67 x 10-3 )(6.67 x 10-3 )2 3.0 x 10-7 Q Ksp a ppte will form
9. Calculate the number of moles of AgCl that will dissolve in 1.0 litre of a 0.10 M CaCl2 solution. (n = 9.0 x 10-10 mol)
- CaCl2 Ca 2 Cl 0.10 0.20 - - 10 AgCl Ag Cl ; Ksp [Ag ][Cl ] 1.8 x 10 ICE x 0.20 (x)(0.20) 1.8 x 1010 ; x 9.0 x 1010 molar solubility 9.0 x 1010 mol n x 1.0 L 9.0 x 1010 AgCl L
-4 -4 10. 50. mL of a 5.0 x 10 M Ca(NO3)2 is mixed with 50. mL of a 2.0 x 10 M NaF to give 100. mL of -11 -12 solution. Will a ppte form? Ksp CaF2 is 3.9 x 10 . (Q = 2.5 x 10 ) NaF Na F- C 2.0 x 10-4 mol/L, V 0.050 L n 1.0 x 10-5mol 2 - Ca(NO3)2 Ca 2 NO3 C 5.0 x 10-4 mol/L, V 0.050 L n 2.5 x 10-5mol 2 - 2 - 2 11 CaF2 Ca 2 F ; Ksp [Ca ][F ] 3.9 x 10 2.5 x 10-5mol 1.0 x 10-5mol
0.100 L 0.100 L 2.5 x 10-4 1.0 x 10-5 Q (2.5 x 10-4)(1.00 x 10-4)2 2.5 x 10-12 Q Ksp no ppte will form
-11 11. Calculate the solubility of RaSO4 in mol/L in a 0.10 M Na2SO4 sol’n. Ksp RaSO4 = 4.3 x 10 . (x = 4.3 x 10-10 M) 2- Na2SO4 2 Na SO4 0.10 2 2- 2 2- 11 RaSO4 Ra SO4 ; Ksp [Ra ][SO4 ] 4.3 x 10 ICE x 0.10 (x)(0.10) 4.3 x 1011 ; x 4.3 x 1010 molar solubility
- -2 + 12. A solution contains 0.010 moles of Cl and 0.0010 moles of CrO4 per litre. Ag is added. -10 -12 + Which will ppte first AgCl or Ag2CrO4. Ksp AgCl = 1.8 x 10 , Ksp Ag2CrO4 = 1.2 x 10 . ([Ag ] - 2- with Cl is smaller than CrO4 , therefore AgCl will form a precipitate first)
2- 2 2- Ag2CrO4 2 Ag CrO4 ; Ksp [Ag ] [CrO4 ] ICE 2x 0.0010 1.2 x 1012 (2x)2(0.0010) 1.2 x 1012 ; x 1.7 x 105 - - AgCl Ag Cl ; Ksp [Ag ][Cl ] ICE x 0.010 1.8 x 1010 (x)(0.010) 1.8 x 1010 ; x 1.8 x 108 as [Ag ] is smaller for AgCl, it will precipitate first.
EQUILIBRIUM REVIEW
1. Two colourless solutions are mixed in a stoppered flask. As the reaction proceeds, the resulting solution turns red, and a colourless gas is formed. After a few minutes, no more gas is evolved but the red colour remains. What evidence is there that equilibrium has been established?
There are no macroscopic changes.
2. What evidence is there to indicate that equilibrium is a dynamic state?
The reaction can be shifted in either direction.
3. Using the Ideal gas law and the formula relating Concentration and moles, derive the relationship that explains why pressure can be considered as a concentration unit for gases.
n P = RT or P = CRT V
4. Write the equilibrium expressions (Ke or K) for each of the following reactions: 2 (a) H + F 2 HF ; [HF] 2 (g) 2 (g) (g) Keq = [H ][F ] (b) 4 NO (g) + 3 O2 (g) 2 N2O5 (g) ; 2 2 2 [N2O5] Keq = (c) BaCO3 (s) BaO (s) + CO2 (g) ; 4 3 [NO] [O2] Keq = [CO2] 2 (d) Fe + Cu2+ Fe2+ + Cu [Fe ] ; (s) (aq) (aq) Keq = (s) [Cu2] (e) HSO - H+ + SO 2- ; 2- 4 (aq) (aq) 4 (aq) [H ][SO4 ] Keq = [HSO -] 2- + 2- - 4[Cr O 2-][OH] (f) 2 CrO4 (aq) + H (aq) Cr2O7 (aq) + OH (aq) ; K = 2 7 eq 2- 2 [CrO4 ] [H ] + 2- (g) Ag2CO3 (s) 2 Ag (aq) + CO3 (aq) ; 2 2- 3 Keq = [Ag ] [CO3 ] 5. The equilibrium constants for three different reactions are:
12 -15 (a) Keq = 1.5 x 10 ; (b) Keq = 0.15; (c) Keq = 4.3 x 10
In which reaction is there: (a) a large ratio of product to reactant? (a) (b) a small ratio of reactant to product? (a)
6. When 0.035 mole of PCl5 is heated to 250°C in a 1-litre vessel, an equilibrium is established in which the concentration of Cl2 is 0.025 mol/L. Find the Keq. [PCl3][Cl2] PCl5 PCl3 Cl2 ; Keq [PCl5] ICE 0.035 - x x 0.025 [PCl3][Cl2] (0.025)(0.025) Keq 0.063 [PCl5] (0.035 - 0.025) 7. Assume that the analysis of another equilibrium mixture of the system in Problem 6 shows that the equilibrium concentration of PCl5 is 0.012 mol/L and that of Cl2 is 0.049 mol/L. What will be the equilibrium concentration of PCl3? The reaction is carried out at 250°C. Use Keq from question 6. [PCl3][Cl2] PCl5 PCl3 Cl2 ; Keq 0.063 [PCl5] ICE 0.012 x 0.049 [PCl3][Cl2] (x)(0.049) Keq 0.063 ; x [PCl3] 0.015 M [PCl5] 0.012
8. How many moles of PCl5 must be heated in a 1-litre flask at 250°C in order to produce enough chlorine to give an equilibrium concentration of 0.10 mol/L? Use Keq from question 6. [PCl3][Cl2] PCl5 PCl3 Cl2 ; Keq 0.063 [PCl5] ICE y - 0.10 0.10 0.10 2 [PCl3][Cl2] (0.10) Keq 0.063 ; 0.063y - 0.0063 0.010 ; y 0.26 [PCl5] y - 0.10
9. Will there be a net reaction when 2.5 moles of PCl5 0.60 mole of Cl2, and 0.60 mole of PCl3 are placed in a 1-litre flask and heated to 250°C? If so, will PCl5 decompose, or will Cl2 and PCl3 react to form more PCl5? Use Keq from question 6. [PCl3][Cl2] PCl5 PCl3 Cl2 ; Keq 0.063 [PCl5] ICE 2.5 - x 0.60 x 0.60 x 0.602 Q 0.144 Q Keq and the rxn will shift to the left until Q Keq 2.5
10. Under a given set of conditions, an equilibrium mixture: SO2 (g) + NO2 (g) SO3 (g) + NO (g) in a 1 litre container was analyzed and found to contain 0.300 mole of SO3, 0.200 mole of NO, 0.050 mole of NO2, and 0.400 mole of SO2. Calculate the Keq.
[SO3][NO] (0.300)(0.200) Keq 3.0 [SO2][NO2] (0.400)(0.050)
11. At 55°C, for the reaction 2 NO2 (g) N2O4 (g) : Keq = 1.15 (a) Write the equilibrium expression. [N O ] K 2 4 1.15 eq 2 [NO2]
(b) Calculate the concentration of N2O4 present in equilibrium with 0.5 mole of NO2. [N O ] x K 2 4 1.15 ; x 0.29 M eq 2 2 [NO2] 0.5 12. Calculate the Keq for the following reaction from the data given below. CO2 (g) + H2 (g) CO (g) + H2O (g) -3 -3 [CO] = [H2O] = 1.33 x 10 mol/L, [CO2] = [H2] = 1.17 x 10 mol/L -3 -3 [CO][H2O] (1.33 x 10 )(1.33 x 10 ) Keq 1.29 [CO2][H2] (1.17 x 10-3)(1.17 x 10-3)
13. One mole of pure NH3 was injected into a 1-litre flask at a certain temperature. The equilibrium mixture below was then analyzed and found to contain 0.300 mol of H2: 2 NH3 N2 + 3 H2 (a) Calculate the concentration of N2 at equilibrium. [N ][H ]3 2 NH N 3 H ; K 2 2 3 2 2 eq 2 [NH3] ICE 1.00 - 2x x 3x 0.300 ; x 0.100 M [N2]
(b) Calculate the concentration of NH3 at equilibrium. [NH3] 1.00 - 2x 1.00 - 0.200 0.80 M
(c) Calculate the equilibrium constant for this system at this temperature and pressure. [N ][H ]3 (0.100)(0.300)3 K 2 2 4.2 x 10-3 eq 2 2 [NH3] 0.80
(d) Which way would the equilibrium be shifted if 0.600 mole of H2 were injected into the flask? The equilibrium will shift to the left, .
(e) How would the injection of hydrogen into the flask affect the equilibrium constant? There would be no change to the equilibrium constant.
(f) How would the equilibrium constant be affected if the pressure of this system were suddenly increased? There would be no change to the equilibrium constant.
14. When 0.50 mole of CO2 and 0.50 mole of H2 were forced into a 1-litre reaction container, the following equilibrium was established: CO2 (g) + H2 (g) H2O (g) + CO (g) and Keq = 2.00. (a) Find the equilibrium concentration of each reactant and product. [H2O][CO] CO2 H2 H2O CO ; Keq 2.00 [CO2][H2] ICE 0.50 - x 0.50 - x x x 2 [H2O][CO] x x x Keq 2.00 ; 2.00 ; 1.4 [CO2][H2] (0.50 x)2 0.50 - x 0.50 - x (0.50 x)(1.4) x ; 0.7 - 1.4 x x ; 2.4 x 0.7 ; x 0.29 [H2O] [CO] 0.29 M and [CO2] [H2] 0.50 - 0.29 0.21 M (b) How would the equilibrium concentrations differ if 0.50 mole of H2O and 0.50 mole of CO had been introduced into the reaction vessel instead of the CO2 and H2? [H2O][CO] CO2 H2 H2O CO ; Keq 2.00 [CO2][H2] ICE x x 0.50 - x 0.50 - x 2 [H2O][CO] (0.50 x) 0.50 - x 0.50 - x Keq 2.00 ; 2.00 ; 1.4 [CO2][H2] x2 x x 0.50 x 1.4 x ; 2.4 x 0.5 ; x 0.21 [H2O] [CO] 0.50 - 0.21 0.29 M and [CO2] [H2] 0.21 M
15. The following equation represents a gaseous system at equilibrium: 2 H2O (g) + heat 2 H2 (g) + O2 (g) Indicate in which direction the equilibrium will shift when the following changes are made:
(a) The concentration of H2 is increased. To the left, . (b) The partial pressure (concentration) of H2O is increased. To the right, . (c) The concentration of O2 is decreased. To the right, . (d) The temperature is increased. To the right, . (e) The total pressure is increased. To the left, .
16. Consider the following reaction:
N2O4 (g) 2 NO2 (g) ; H = +ve ; Keq = 0.87 at 55 C What will be the effect of each of the following changes on the concentration of N2O4 at equilibrium:
(a) increasing the pressure. The [N2O4] will until Keq is reached again. (b) increasing the temperature. The [N2O4] will until Keq is reached again. (c) increasing the volume. The [N2O4] will until Keq is reached again. (d) adding more NO2 (g) to the system without changing P or T. The [N2O4] will . (e) adding a catalyst? no change
17. Answer the same questions (a,b,c,e) for the following reaction as you did for the reaction given in Problem 16 for the concentration of water. 40 H2 (g) + 1/2 O2 (g) H2O (g) ; H = -ve ; Keq = 1.0 x 10 at 25 C (a) [H2O] will (b) [H2O] will (c) [H2O] will (e) no change
18. How can you increase the concentration of the product in each of the following reactions by varying the temperature and pressure?
(a) 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) ; H = -ve
(i) T (ii) P
(b) Br2 (g) + Cl2 (g) 2 BrCl (g) ; H = +ve (i) T (ii) P no change
2+ 2- (c) BaSO4 (s) Ba (aq) + SO4 (aq) ; H = +ve (i) T (ii) P no change 19. Write the equation for the chemical equilibrium that exists between undissolved and dissolved solute in a saturated solution for each of the following slightly soluble sulfides. -24 -35 T12S Ksp = 1 x 10 CuS Ksp = 1 x 10 + 2- 2+ 2- T12S 2 Tl + S CuS Cu + S -48 -52 Cu2S Ksp = 1 x 10 HgS Ksp = 1 x 10 + 2- 2+ 2- Cu2S 2 Cu + S HgS Hg + S Rank each of these sulfides in order of decreasing molar solubility in their saturated solutions. T12S > CuS > Cu2S > HgS
20. 500 mL of a saturated solution of silver carbonate, Ag2CO3, is evaporated to dryness leaving 0.0698 g of Ag2CO3. What is the Ksp of silver carbonate? 1 mol Ag2CO3 -4 mAg CO 0.0698 g ; n 0.0698 g x 2.531 x 10 mol 2 3 275.75 g 2.531 x 10-4 mol then C 5.063 x 10-4 M 0.500 L 2- 2 2- Ag2CO3 2 Ag CO3 ; Ksp [Ag ] [CO3 ] ICE 2(5.063 x 10-4) 5.063 x 10-4 2 2- -4 2 -4 -10 Ksp [Ag ] [CO3 ] (1.013 x 10 ) (5.063 x 10 ) 5.190 x 10 21. What mass of barium fluoride, BaF2, will dissolve in 500. mL of a 0.100 M NaF solution? The Ksp of barium fluoride is 1.7 x 10-6. Fluoride ion is the common ion. NaF Na F- 0.100 2 - 2 - 2 6 BaF2 Ba 2 F ; Ksp [Ba ][F ] 1.7 x 10 ICE x 0.100 (x)(0.100)2 1.7 x 106 ; x 1.7 x 104 molar solubility and m 1.5 x 102 g BaF2 2+ - 22. (a) Will a precipitate of BaF2 form when 0.035 mol of Ba and 0.015 mol of F are combined in 1.000 L of solution? 2 - 2 - 2 6 BaF2 Ba 2 F ; Ksp [Ba ][F ] 1.7 x 10 2 6 Q (0.035)(0.015) 7.9 x 10 ; Q Ksp a ppte will form Here is the same question, but asked in an alternate way: Will a precipitate of BaF2 form when the following solutions are mixed? -6. 700. mL of 0.050 M BaC12 + 300. mL of 0.050 M NaF Ksp(BaF2) = 1.7 x 10 NaF Na F- C 0.050 M ; V 0.300L then n 0.015 mol 2 - BaCl2 Ba 2 Cl C 0.050 M ; V 0.700L then n 0.035 mol 2 - 2 - 2 6 BaF2 Ba 2 F ; Ksp [Ba ][F ] 1.7 x 10 0.035 mol 0.015 mol
1.0 L 1.0 L 2 6 Q (0.035)(0.015) 7.9 x 10 ; Q Ksp a ppte will form Extra SCH 4U Review Questions
-5 1. What is the solubility of PbCl2 in 0.10 M NaCl? (Ksp PbCl2 is 1.2 x 10 ).
NaCl Na1 Cl1- 0.10 M 2 - 2 - 2 PbCl2 Pb (aq) 2 Cl (aq) ; Ksp [Pb ][Cl ] ICE x 0.10 1.2 x 105 2 - 2 5 2 5 5 Ksp [Pb ][Cl ] 1.2 x 10 ; (x)(0.10) 1.2 x 10 ; 0.01x 1.2 x 10 x 1.2 x 103 [Pb2 ] molar solubility then, solubility of PbCl molar solubility x M 2 PbCl2 1.2 x 103 mol PbCl 278.10 g 2 x 0.33 g/L L mol PbCl2
2. What is the Keq for A + 2B AB2 if [AB2]i = 0.20 M and [A]eq = 0.050 M?
[AB2 ] A 2B AB2 ; Keq [A][B]2 ICE x 2x 0.20 - x 0.050
[AB2] (0.20 0.050) 0.15 Keq 300 [A][B]2 (0.050)[2(0.050)]2 0.0005 3. If 1.00 g of NaCl(s) is added to 500. mL of a 0.010 M Pb(NO3)2 will a precipitate form? (Ksp PbCl2 = 1.2 x 10-5).
NaCl Na Cl- 1mol NaCl 1 M 58.44 g/mol 1.00 g x x 3.42 x 10-2 mol/L 58.44 g 0.500 L 2 - Pb(NO3)2 Pb 2NO3
0.010 2 - 2 - 2 PbCl2 Pb (aq) 2 Cl (aq) ; Ksp [Pb ][Cl ] Q (0.010)(3.42 x 10-2 )2 1.2 x 10-5 Q Ksp no ppte will form
4. What is the [AB]eq in A + B AB if [A]i = [B]i = 0.10 M (Keq = 101).
[AB] A B AB ; K 101 eq [A][B] ICE 0.10 - x 0.10 - x x 100 Rule - doesn' t apply [AB] (x) K 101 eq [A][B] (0.10 - x)2 x 1.01 - 20.2 x 101x2 101x2 - 21.2x 1.01 0 21.2 449.44 - 408.04 21.2 6.43 x 202 202 x 0.14 or x 0.073 [A]eq [B]eq 0.10 - 0.073 0.027 and [AB]eq 0.073 0.073 Check : Q 100 Q Keq (0.027)2
-12 5. What is the solubility in g/100 mL of Ag2CO3 if the Ksp for Ag2CO3 is 8.5 x 10 ? -2 2 -2 Ag2CO3 2 Ag (aq) CO3 (aq) ; Ksp [Ag ] [CO3 ] ICE 2x x 8.5 x 1012 2 -2 12 2 12 3 12 Ksp [Ag ] [CO3 ] 8.5 x 10 ; (2x) (x) 8.5 x 10 ; 4x 8.5 x 10 4 -2 x 1.29 x 10 [CO3 ] molar solubility then, solubility of Ag CO molar solubility x M 2 3 Ag2CO3 1.29 x 104 mol Ag CO 275.75 g 0.1 L 2 3 x x 0.0036 g/100 mL L mol Ag2CO3 100 mL