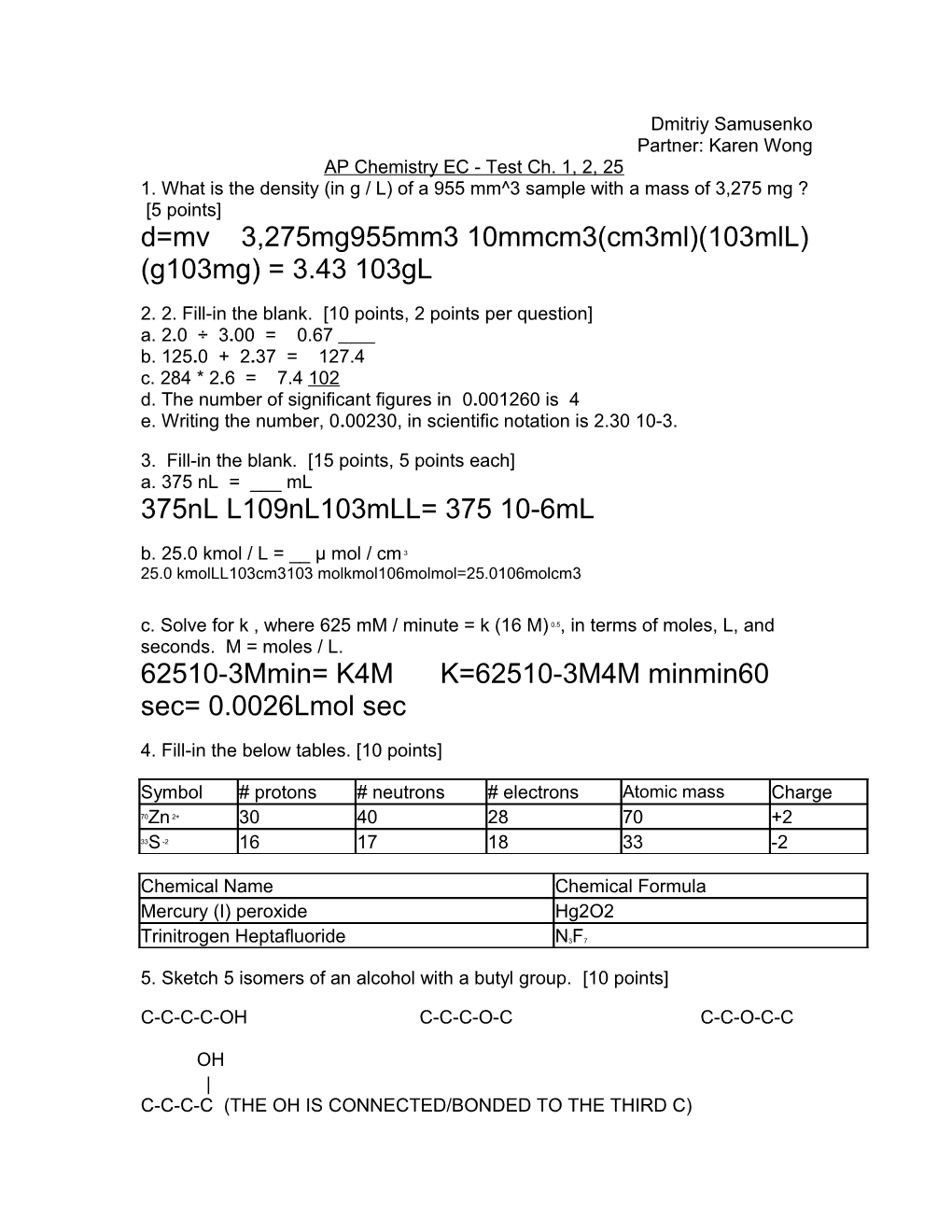
Dmitriy Samusenko Partner: Karen Wong AP Chemistry EC - Test Ch. 1, 2, 25 1. What is the density (in g / L) of a 955 mm^3 sample with a mass of 3,275 mg ? [5 points] d=mv 3,275mg955mm3 10mmcm3(cm3ml)(103mlL) (g103mg) = 3.43 103gL
2. 2. Fill-in the blank. [10 points, 2 points per question] a. 2.0 ÷ 3.00 = 0.67 b. 125.0 + 2.37 = 127.4 c. 284 * 2.6 = 7.4 102 d. The number of significant figures in 0.001260 is 4 e. Writing the number, 0.00230, in scientific notation is 2.30 10-3.
3. Fill-in the blank. [15 points, 5 points each] a. 375 nL = ___ mL 375nL L109nL103mLL= 375 10-6mL b. 25.0 kmol / L = __ µ mol / cm 3 25.0 kmolLL103cm3103 molkmol106molmol=25.0106molcm3 c. Solve for k , where 625 mM / minute = k (16 M) 0.5, in terms of moles, L, and seconds. M = moles / L. 62510-3Mmin= K4M K=62510-3M4M minmin60 sec= 0.0026Lmol sec
4. Fill-in the below tables. [10 points]
Symbol # protons # neutrons # electrons Atomic mass Charge 70Zn 2+ 30 40 28 70 +2 33S -2 16 17 18 33 -2
Chemical Name Chemical Formula Mercury (I) peroxide Hg2O2
Trinitrogen Heptafluoride N3F7
5. Sketch 5 isomers of an alcohol with a butyl group. [10 points]
C-C-C-C-OH C-C-C-O-C C-C-O-C-C
OH | C-C-C-C (THE OH IS CONNECTED/BONDED TO THE THIRD C) C | C-C-C-OH (THE TOP C IS CONNECTED TO THE SECOND C) and etc.
6. Sketch: [15 points] a. aldehyde with methyl group(s) O || H3C- C - H b. amine with propyl group(s) H2N - C3H7 c. Carboxylic acid with ethyl group(s) C2H5-COOH
7. Sketch / label a peptide bond between two amino acids. [5 points] R O R O | || | || H2N- C - C - N - C - C - OH | | | H H H THE STUDENT ALSO HAS THE OPTION OF INVERTING THE SKETCH AT THE MIDDLE N FOR EQUAL CREDIT.
********************************************************************** Ralph Ng AP Chemistry 5° Mr. Matsumoto 5/15/15 Answer Key for Tests(E.C.) Note: Answers are underlined and/or bold-faced. 1) Chapter 1, 2, & 25(Partners: Ralph, Dmitriy & Karen)
1. What is the density(in g/L) of a 955 mm^3 sample with a mass of 3.275 mg? d= m/v = 3,275 mg/(955 mm^3)(10mm/cm)^3(cm^3/mL)(10^3mL/L)(g/10^3mg)
= 3.43x10^3 g/L
2. Fill in the blank. a. 2.0 / 3.00 = 0.67
b. 125.0 + 2.37 = 127.4
c. 284 x 2.6 = 7.4x10^2
d. The number of significant figures in 0.001260 is 4.
e. Writing the number, 0.00230, in scientific notation is 2.30 x 10^-3
3. Fill in the blank.
a. 375 nL = ____mL 375nL(L/10^9nL)(10^3mL/L) = 375 x 10^-6 mL
b. 25.0 kilomoles/L = ___ µmoles/cm^3 25.0 kmol/L(L/10^3cm^3)(10^3mol/kmol)(10^6 µmol/1 mol) = 25.0x10^6 µmoles/cm^3
c. Solve for k, where 625 mM/minute = k(16M)^.5, in terms of moles, L, and seconds. M=moles/L 625x10^-3M/min = k(4 √M) K = 625x10^-3M/(4 √M min)(min/60sec) = 0.0026√L/ ( √M sec)
4. Fill in the below tables. Symbol # protons # neutrons #electrons Atomic Charge mass 70Zn2+ 30 40 28 70 +2
33S-2 16 17 18 33 -2
Chemical name Chemical formula Mercury(I) peroxide Hg2O2 Trinitrogen heptafluoride N3F7
5. Sketch 5 isomers of an alcohol with a butyl group. C-C-C-C-OH
C-C-C-O-C
C-C-O-C-C
OH
C-C-C-C
C
C – C – C – OH
6. Sketch:
a. Aldehyde with methyl group(s)
O H3C – C – H
b. Amine with propyl group(s) H2N – C3H7
c. Carboxylic acid with ethyl group(s) C2H5 – COOH
7. Sketch/label a peptide bond between two amino acids.
R O R’ O H3N – C – C – N – C – C- OH H H H Peptide bond