1
Supporting Online Information
Mutagenesis alters the catalytic mechanism of the light-driven enzyme protochlorophyllide oxidoreductase
Binuraj R. K. Menon, Paul A. Davison, C. Neil Hunter, Nigel S. Scrutton & Derren J. Heyes
Supplementary tables
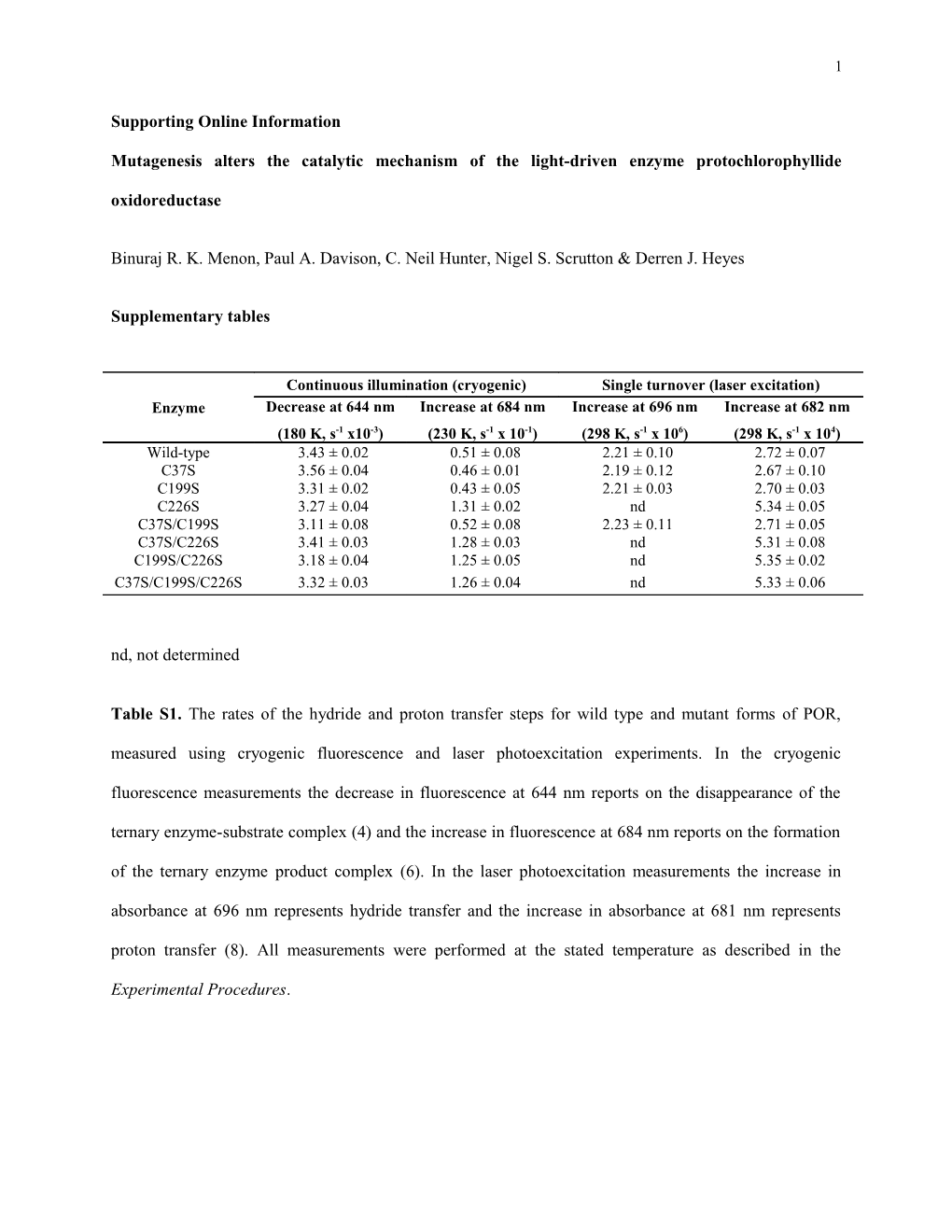
Continuous illumination (cryogenic) Single turnover (laser excitation) Enzyme Decrease at 644 nm Increase at 684 nm Increase at 696 nm Increase at 682 nm (180 K, s-1 x10-3) (230 K, s-1 x 10-1) (298 K, s-1 x 106) (298 K, s-1 x 104) Wild-type 3.43 ± 0.02 0.51 ± 0.08 2.21 ± 0.10 2.72 ± 0.07 C37S 3.56 ± 0.04 0.46 ± 0.01 2.19 ± 0.12 2.67 ± 0.10 C199S 3.31 ± 0.02 0.43 ± 0.05 2.21 ± 0.03 2.70 ± 0.03 C226S 3.27 ± 0.04 1.31 ± 0.02 nd 5.34 ± 0.05 C37S/C199S 3.11 ± 0.08 0.52 ± 0.08 2.23 ± 0.11 2.71 ± 0.05 C37S/C226S 3.41 ± 0.03 1.28 ± 0.03 nd 5.31 ± 0.08 C199S/C226S 3.18 ± 0.04 1.25 ± 0.05 nd 5.35 ± 0.02 C37S/C199S/C226S 3.32 ± 0.03 1.26 ± 0.04 nd 5.33 ± 0.06
nd, not determined
Table S1. The rates of the hydride and proton transfer steps for wild type and mutant forms of POR, measured using cryogenic fluorescence and laser photoexcitation experiments. In the cryogenic fluorescence measurements the decrease in fluorescence at 644 nm reports on the disappearance of the ternary enzyme-substrate complex (4) and the increase in fluorescence at 684 nm reports on the formation of the ternary enzyme product complex (6). In the laser photoexcitation measurements the increase in absorbance at 696 nm represents hydride transfer and the increase in absorbance at 681 nm represents proton transfer (8). All measurements were performed at the stated temperature as described in the
Experimental Procedures. 2
Wild type C226S
H2O D2O H2O D2O
∆H‡ (kJ mol-1) 53.7 ± 1.6 69.3 ± 2.6 36.6 ± 1.8 36.2 ± 2.3
∆∆H‡ (kJ mol-1) 15.6 ± 3.1 0.4 ± 2.9
∆S‡ (J mol-1 K-1) 19.4 ± 0.5 65.8 ± 2.1 -32.3 ± 1.2 -43.4 ± 2.2
ln A’ 26.1 ± 0.6 31.7 ± 1.0 19.9 ± 0.7 18.5 ± 0.9
A’H/A’D 0.004 ± 0.005 4.1 ± 2.6
Table S2. Thermodynamic parameters for the formation of Chlide in wild type POR and the C226S variant. The enthalpies of activation, ∆H‡, and the entropies of activation, ∆S‡, have been calculated by fitting the temperature dependence data to the Eyring equation for each step. 3
Supplementary figures
Figure S1. 77 K absorbance difference spectra of
10 μM Pchlide and 250 μM NADPH in the
presence of 42.3 μM C226S variant enzyme,
recorded after illumination for 10 mins at
different temperatures ranging from 77 K to 280
K. The difference spectra were obtained by using a non-illuminated sample as a blank. The arrows indicate the formation and disappearance of the different absorbance bands at higher temperatures. 4
Figure S2. Cryogenic absorbance spectra showing latter stages of Chlide formation in wild type POR and the C226S variant. 77 K absorbance spectra of 10 μM Pchlide and 250 μM NADPH in the presence of either 42.3 μM C226S variant enzyme (A) or 20 μM wild type POR (B), recorded after illumination for 10 mins at different temperatures ranging from 205 K to 280 K. The arrows indicate the formation and disappearance of the different absorbance bands at higher temperatures. 5
Figure S3. Typical kinetic traces measured at 681 nm over 180 μs for the wild type (black) and C226S variant (red) following photoexcitation with a 6 ns laser pulse at 450 nm. All traces were collected at 25°C as described in the Experimental Procedures section. Samples contained 15 µM Pchlide and 250 µM
NADPH in the presence of 50.0 µM C37S, C199S or C33S/C199S. Higher concentrations of enzyme were used for the C37S/C226S (117.9 µM), C199S/C226S (119.2 µM) and C37SC/199S/C226S (105.3 µM) variant enzymes, based on the dissociation constant of Pchlide to ensure that equivalent ternary complex concentrations were used in the laser excitation studies. 6
Figure S4. Typical kinetic traces measured at 681 nm over 180 μs for the C226S variant following photoexcitation with a 6 ns laser pulse at 450 nm. The transients were measured in the presence of either
2 NADPH or NADP H (NADPD) and in different combinations of H2O and D2O buffer. Samples contained
15 μM Pchlide and 250 μM NADPH/D in the presence of either 92.2 μM C226S.