SCH3U Name:______Date: ______
Lab: Testing the Activity Series
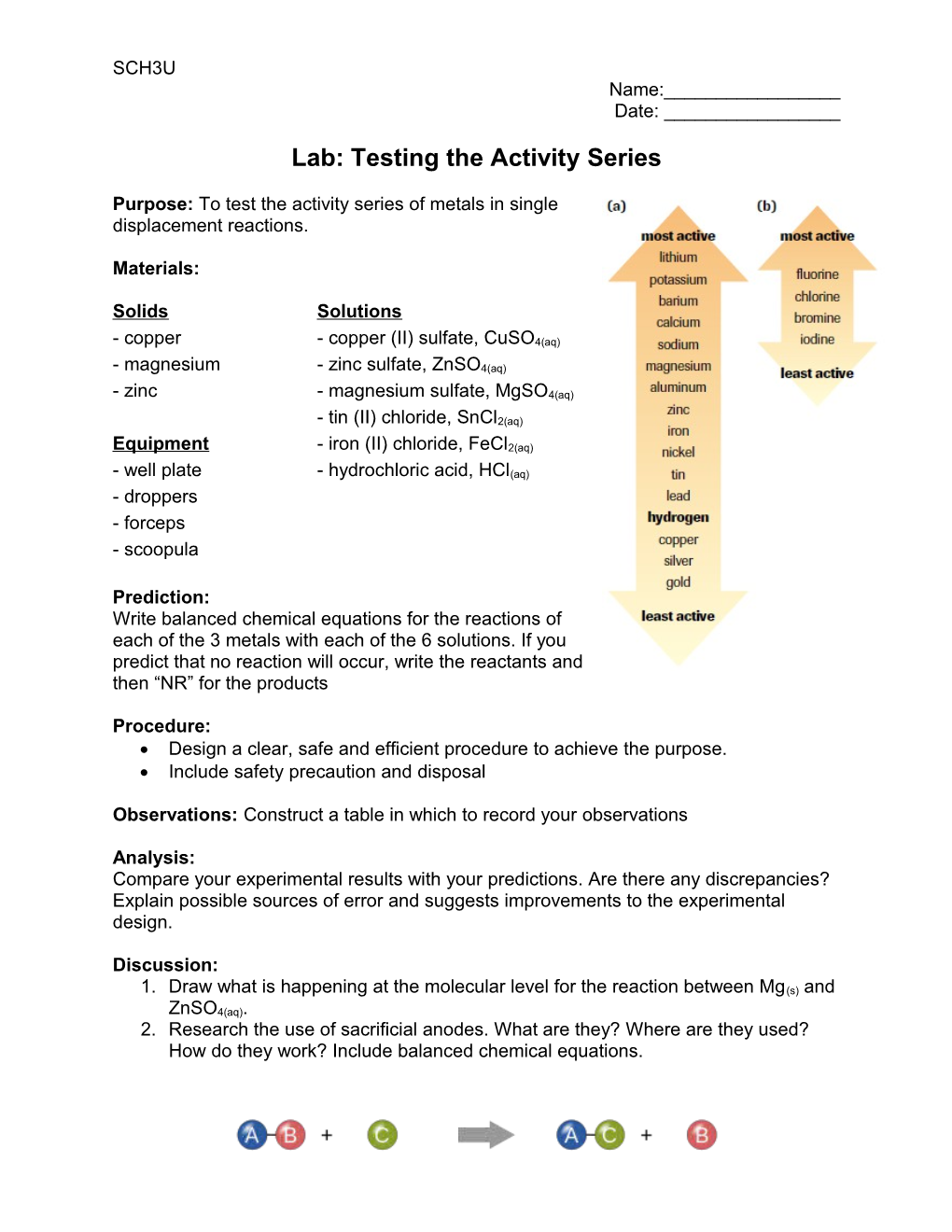
Purpose: To test the activity series of metals in single displacement reactions.
Materials:
Solids Solutions
- copper - copper (II) sulfate, CuSO4(aq)
- magnesium - zinc sulfate, ZnSO4(aq)
- zinc - magnesium sulfate, MgSO4(aq)
- tin (II) chloride, SnCl2(aq)
Equipment - iron (II) chloride, FeCl2(aq)
- well plate - hydrochloric acid, HCl(aq) - droppers - forceps - scoopula
Prediction: Write balanced chemical equations for the reactions of each of the 3 metals with each of the 6 solutions. If you predict that no reaction will occur, write the reactants and then “NR” for the products
Procedure: Design a clear, safe and efficient procedure to achieve the purpose. Include safety precaution and disposal
Observations: Construct a table in which to record your observations
Analysis: Compare your experimental results with your predictions. Are there any discrepancies? Explain possible sources of error and suggests improvements to the experimental design.
Discussion: 1. Draw what is happening at the molecular level for the reaction between Mg(s) and ZnSO4(aq). 2. Research the use of sacrificial anodes. What are they? Where are they used? How do they work? Include balanced chemical equations.