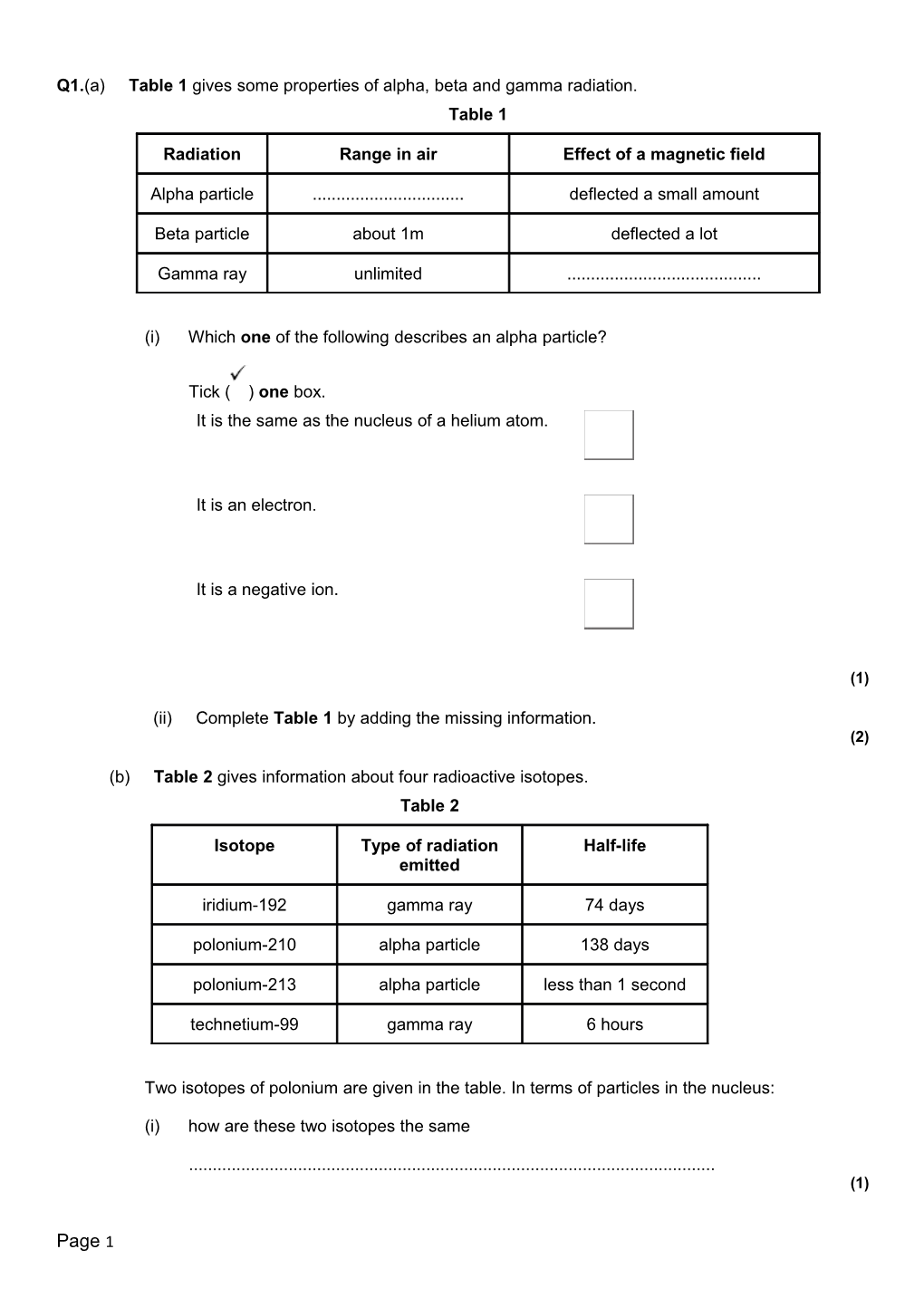
Q1.(a) Table 1 gives some properties of alpha, beta and gamma radiation. Table 1
Radiation Range in air Effect of a magnetic field
Alpha particle ...... deflected a small amount
Beta particle about 1m deflected a lot
Gamma ray unlimited ......
(i) Which one of the following describes an alpha particle?
Tick ( ) one box. It is the same as the nucleus of a helium atom.
It is an electron.
It is a negative ion.
(1)
(ii) Complete Table 1 by adding the missing information. (2)
(b) Table 2 gives information about four radioactive isotopes. Table 2
Isotope Type of radiation Half-life emitted
iridium-192 gamma ray 74 days
polonium-210 alpha particle 138 days
polonium-213 alpha particle less than 1 second
technetium-99 gamma ray 6 hours
Two isotopes of polonium are given in the table. In terms of particles in the nucleus:
(i) how are these two isotopes the same
...... (1)
Page 1 (ii) how are these two isotopes different?
...... (1)
(c) To monitor the blood flow through a patientߣ s heart, a doctor injects the patient with a very small dose of technetium-99. The gamma radiation detected outside of the patient’s body allows the doctor to see if the heart is working correctly.
(i) Explain why technetium-99 is more suitable for this use than polonium-210.
......
......
......
...... (2)
(ii) Explain why technetium-99 is more suitable for this use than iridium-192.
......
......
......
...... (2)
(d) Technetium-99 (Tc) is produced by the beta decay (β) of an isotope of molybdenum (Mo).
The decay can be represented by the equation below.
Complete the equation by writing the correct number in each of the two boxes.
(2) (Total 11 marks)
(e) When a thorium-230 nucleus decays, it emits radiation and changes into radium-226.
What type of radiation, alpha, beta or gamma, is emitted by thorium-230?
......
Explain the reason for your answer.
Page 2 ......
......
......
......
...... (3)
M1.(a) (i) It is the same as the nucleus of a helium atom. 1
(ii) (about) 5 cm accept any number between 2 and 8 cm 1
not deflected accept none 1
(b) (i) number of protons accept same atomic / proton number 1
(ii) numbers of neutrons accept different mass numbers 1
(c) (i) because polonium-210 is an alpha emitter 1
and alpha particles cannot be detected outside bodyor alpha particles produce heavy ionisation 1
(ii) because iridium-192 has a long(er) half life 1
and so will be radioactive for longer 1 Page 3 (d) 99 1
42 1 [11]
(e) alpha (particle) reason may score even if beta or gamma is chosen 1
mass number goes down by 4or number of protons and neutrons goes down by 4 or number of neutrons goes down by 2 candidates that answer correctly in terms of why gamma and beta decay are not possible gain full credit 1
atomic / proton number goes down by 2or number of protons goes down by 2 accept an alpha particle consists of 2 neutrons and 2 protons for 1 mark
accept alpha equals 42He or 42α for 1 mark an alpha particle is a helium nucleus is insufficient for this mark 1 [8]
Page 4