NATIONAL INSTITUTE OF BIOLOGICALS (Ministry of Health & Family Welfare)
Biological Products Test Reports Released
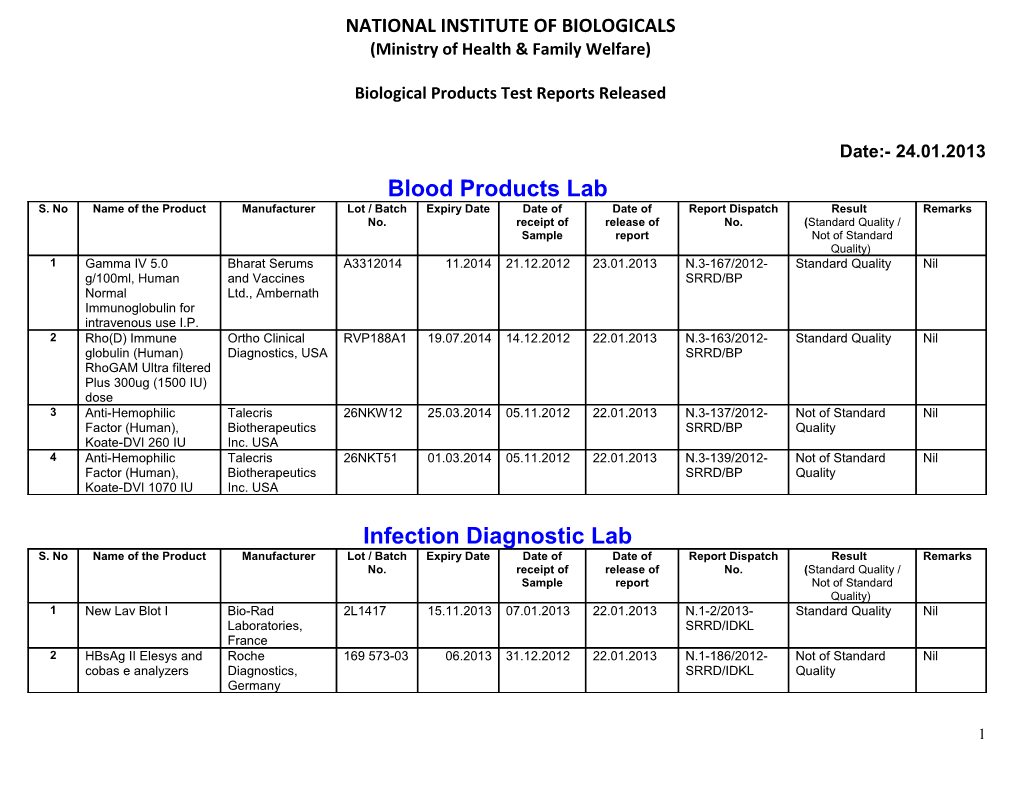
Date:- 24.01.2013 Blood Products Lab S. No Name of the Product Manufacturer Lot / Batch Expiry Date Date of Date of Report Dispatch Result Remarks No. receipt of release of No. (Standard Quality / Sample report Not of Standard Quality) 1 Gamma IV 5.0 Bharat Serums A3312014 11.2014 21.12.2012 23.01.2013 N.3-167/2012- Standard Quality Nil g/100ml, Human and Vaccines SRRD/BP Normal Ltd., Ambernath Immunoglobulin for intravenous use I.P. 2 Rho(D) Immune Ortho Clinical RVP188A1 19.07.2014 14.12.2012 22.01.2013 N.3-163/2012- Standard Quality Nil globulin (Human) Diagnostics, USA SRRD/BP RhoGAM Ultra filtered Plus 300ug (1500 IU) dose 3 Anti-Hemophilic Talecris 26NKW12 25.03.2014 05.11.2012 22.01.2013 N.3-137/2012- Not of Standard Nil Factor (Human), Biotherapeutics SRRD/BP Quality Koate-DVI 260 IU Inc. USA 4 Anti-Hemophilic Talecris 26NKT51 01.03.2014 05.11.2012 22.01.2013 N.3-139/2012- Not of Standard Nil Factor (Human), Biotherapeutics SRRD/BP Quality Koate-DVI 1070 IU Inc. USA
Infection Diagnostic Lab S. No Name of the Product Manufacturer Lot / Batch Expiry Date Date of Date of Report Dispatch Result Remarks No. receipt of release of No. (Standard Quality / Sample report Not of Standard Quality) 1 New Lav Blot I Bio-Rad 2L1417 15.11.2013 07.01.2013 22.01.2013 N.1-2/2013- Standard Quality Nil Laboratories, SRRD/IDKL France 2 HBsAg II Elesys and Roche 169 573-03 06.2013 31.12.2012 22.01.2013 N.1-186/2012- Not of Standard Nil cobas e analyzers Diagnostics, SRRD/IDKL Quality Germany
1