Supplemental content Table
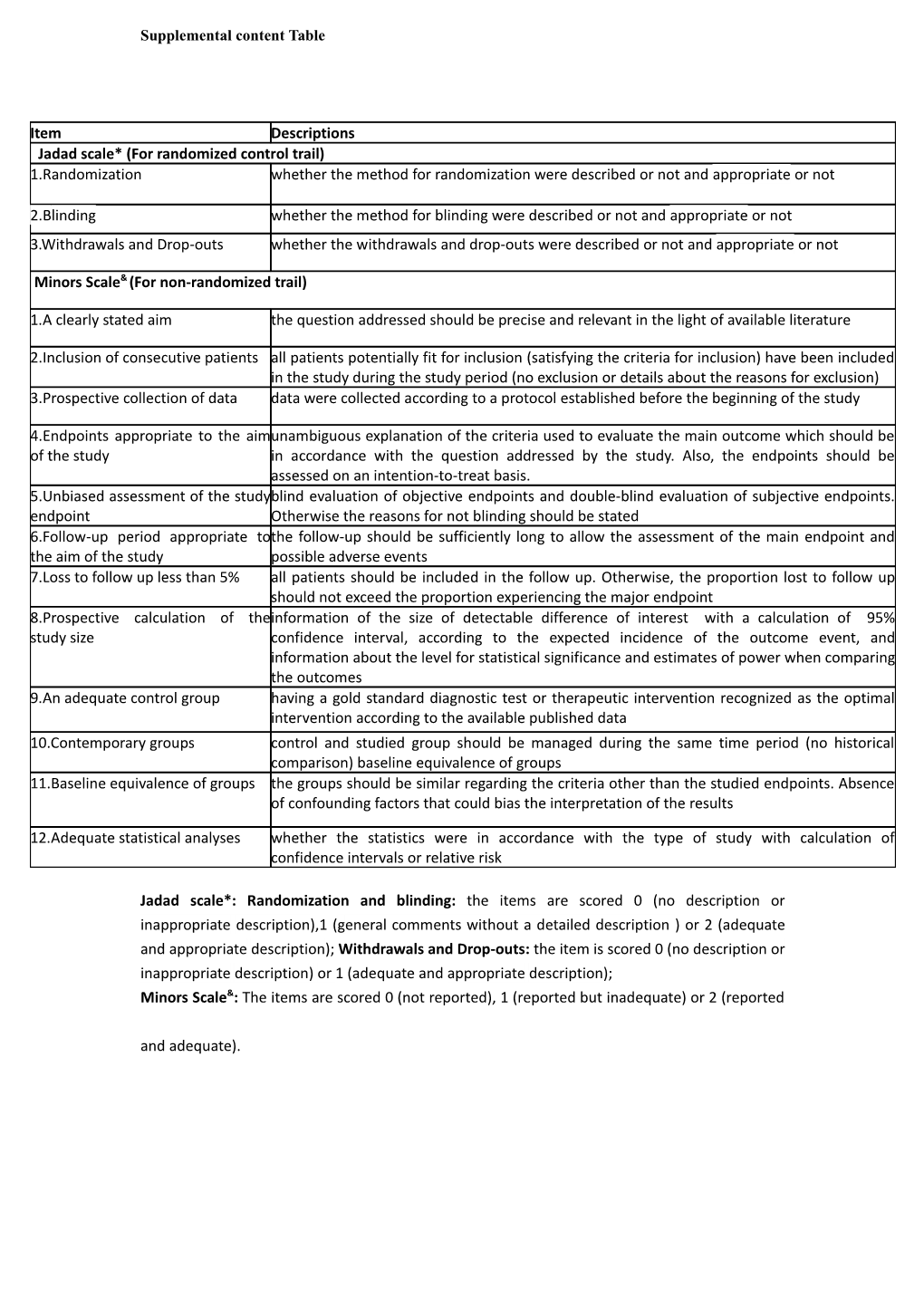
Item Descriptions Jadad scale* (For randomized control trail) 1.Randomization whether the method for randomization were described or not and appropriate or not
2.Blinding whether the method for blinding were described or not and appropriate or not 3.Withdrawals and Drop-outs whether the withdrawals and drop-outs were described or not and appropriate or not
Minors Scale& (For non-randomized trail)
1.A clearly stated aim the question addressed should be precise and relevant in the light of available literature
2.Inclusion of consecutive patients all patients potentially fit for inclusion (satisfying the criteria for inclusion) have been included in the study during the study period (no exclusion or details about the reasons for exclusion) 3.Prospective collection of data data were collected according to a protocol established before the beginning of the study
4.Endpoints appropriate to the aimunambiguous explanation of the criteria used to evaluate the main outcome which should be of the study in accordance with the question addressed by the study. Also, the endpoints should be assessed on an intention-to-treat basis. 5.Unbiased assessment of the studyblind evaluation of objective endpoints and double-blind evaluation of subjective endpoints. endpoint Otherwise the reasons for not blinding should be stated 6.Follow-up period appropriate tothe follow-up should be sufficiently long to allow the assessment of the main endpoint and the aim of the study possible adverse events 7.Loss to follow up less than 5% all patients should be included in the follow up. Otherwise, the proportion lost to follow up should not exceed the proportion experiencing the major endpoint 8.Prospective calculation of theinformation of the size of detectable difference of interest with a calculation of 95% study size confidence interval, according to the expected incidence of the outcome event, and information about the level for statistical significance and estimates of power when comparing the outcomes 9.An adequate control group having a gold standard diagnostic test or therapeutic intervention recognized as the optimal intervention according to the available published data 10.Contemporary groups control and studied group should be managed during the same time period (no historical comparison) baseline equivalence of groups 11.Baseline equivalence of groups the groups should be similar regarding the criteria other than the studied endpoints. Absence of confounding factors that could bias the interpretation of the results
12.Adequate statistical analyses whether the statistics were in accordance with the type of study with calculation of confidence intervals or relative risk
Jadad scale*: Randomization and blinding: the items are scored 0 (no description or inappropriate description),1 (general comments without a detailed description ) or 2 (adequate and appropriate description); Withdrawals and Drop-outs: the item is scored 0 (no description or inappropriate description) or 1 (adequate and appropriate description); Minors Scale&: The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported
and adequate). Supplemental content
Supplementary Figure 1. Detailed search histories for Pubmed. Supplemental contents
Supplementary Figure 2. Detailed search histories forOVID.
Supplemental content Supplementary Figure 3. Detailed search histories for Web of Science databases.