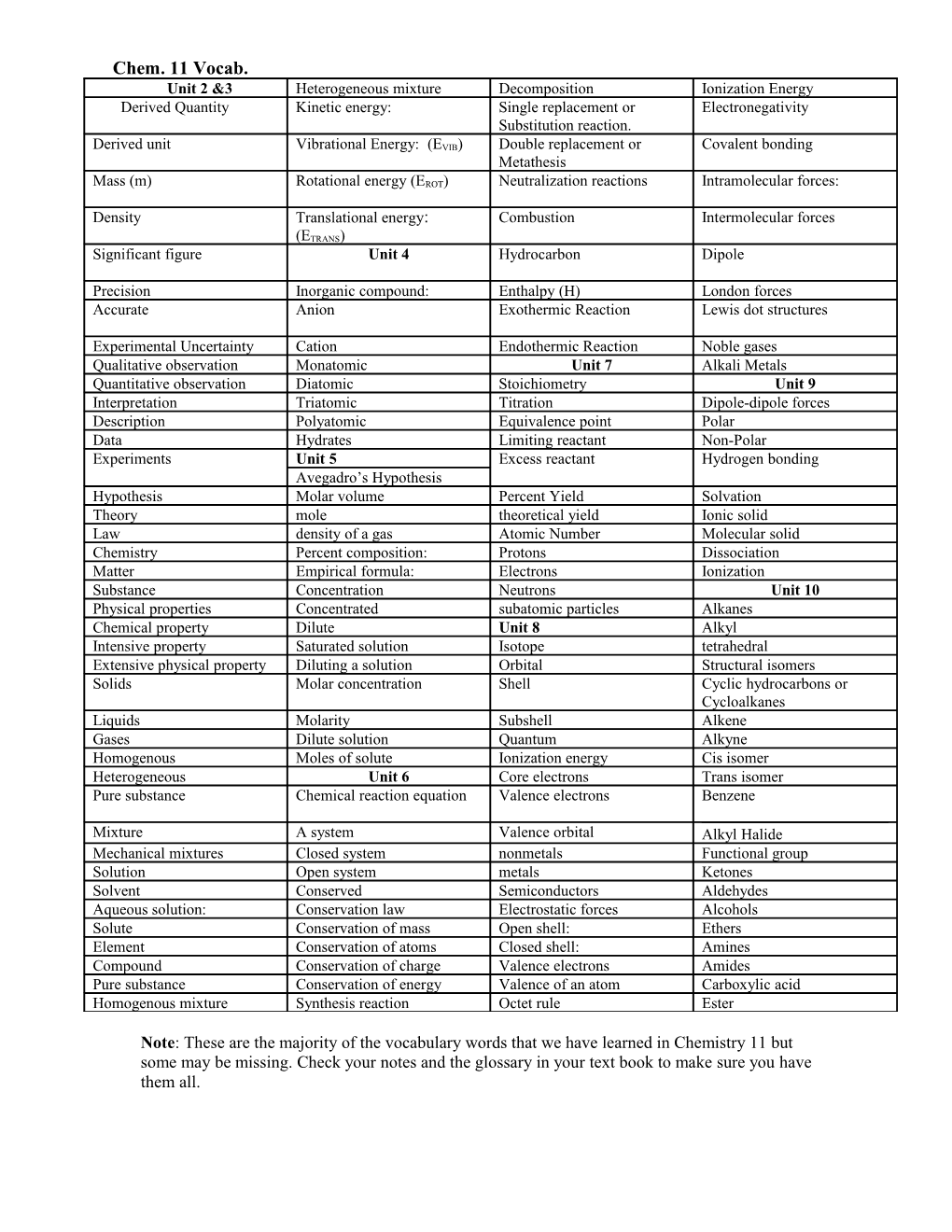
Chem. 11 Vocab. Unit 2 &3 Heterogeneous mixture Decomposition Ionization Energy Derived Quantity Kinetic energy: Single replacement or Electronegativity Substitution reaction.
Derived unit Vibrational Energy: (EVIB) Double replacement or Covalent bonding Metathesis
Mass (m) Rotational energy (EROT) Neutralization reactions Intramolecular forces:
Density Translational energy: Combustion Intermolecular forces (ETRANS) Significant figure Unit 4 Hydrocarbon Dipole
Precision Inorganic compound: Enthalpy (H) London forces Accurate Anion Exothermic Reaction Lewis dot structures
Experimental Uncertainty Cation Endothermic Reaction Noble gases Qualitative observation Monatomic Unit 7 Alkali Metals Quantitative observation Diatomic Stoichiometry Unit 9 Interpretation Triatomic Titration Dipole-dipole forces Description Polyatomic Equivalence point Polar Data Hydrates Limiting reactant Non-Polar Experiments Unit 5 Excess reactant Hydrogen bonding Avegadro’s Hypothesis Hypothesis Molar volume Percent Yield Solvation Theory mole theoretical yield Ionic solid Law density of a gas Atomic Number Molecular solid Chemistry Percent composition: Protons Dissociation Matter Empirical formula: Electrons Ionization Substance Concentration Neutrons Unit 10 Physical properties Concentrated subatomic particles Alkanes Chemical property Dilute Unit 8 Alkyl Intensive property Saturated solution Isotope tetrahedral Extensive physical property Diluting a solution Orbital Structural isomers Solids Molar concentration Shell Cyclic hydrocarbons or Cycloalkanes Liquids Molarity Subshell Alkene Gases Dilute solution Quantum Alkyne Homogenous Moles of solute Ionization energy Cis isomer Heterogeneous Unit 6 Core electrons Trans isomer Pure substance Chemical reaction equation Valence electrons Benzene
Mixture A system Valence orbital Alkyl Halide Mechanical mixtures Closed system nonmetals Functional group Solution Open system metals Ketones Solvent Conserved Semiconductors Aldehydes Aqueous solution: Conservation law Electrostatic forces Alcohols Solute Conservation of mass Open shell: Ethers Element Conservation of atoms Closed shell: Amines Compound Conservation of charge Valence electrons Amides Pure substance Conservation of energy Valence of an atom Carboxylic acid Homogenous mixture Synthesis reaction Octet rule Ester
Note: These are the majority of the vocabulary words that we have learned in Chemistry 11 but some may be missing. Check your notes and the glossary in your text book to make sure you have them all.