Facility level data collection form
Global Indicators
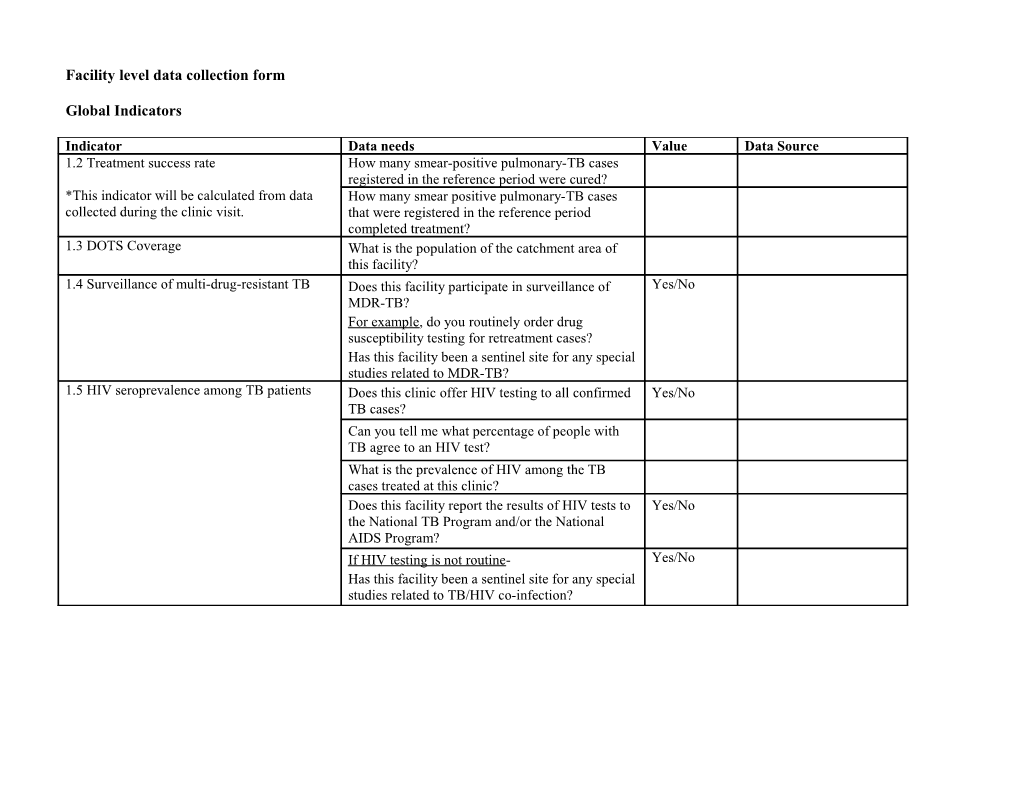
Indicator Data needs Value Data Source 1.2 Treatment success rate How many smear-positive pulmonary-TB cases registered in the reference period were cured? *This indicator will be calculated from data How many smear positive pulmonary-TB cases collected during the clinic visit. that were registered in the reference period completed treatment? 1.3 DOTS Coverage What is the population of the catchment area of this facility? 1.4 Surveillance of multi-drug-resistant TB Does this facility participate in surveillance of Yes/No MDR-TB? For example, do you routinely order drug susceptibility testing for retreatment cases? Has this facility been a sentinel site for any special studies related to MDR-TB? 1.5 HIV seroprevalence among TB patients Does this clinic offer HIV testing to all confirmed Yes/No TB cases? Can you tell me what percentage of people with TB agree to an HIV test? What is the prevalence of HIV among the TB cases treated at this clinic? Does this facility report the results of HIV tests to Yes/No the National TB Program and/or the National AIDS Program? If HIV testing is not routine- Yes/No Has this facility been a sentinel site for any special studies related to TB/HIV co-infection? Facility level data collection form
Routinely reported program outcomes - Case detection Use the most recent quarter for which case detection for smear-positive pulmonary TB is complete.
Quarter 1 (January-March) 2 (April-June) 3 (July-September) 4 (October-December)
Year ______Population of catchment area ______
Indicator Data needs Value Data Source 2.1 Case notification rate, new and relapse How many new TB cases were registered during cases the last quarter? How many relapse cases were registered during the last quarter? 2.2 Case notification rate, smear-positive How many smear-positive TB cases were pulmonary-TB cases registered during the last quarter? 2.3 New pulmonary cases with no smear How many pulmonary cases were registered in the results last quarter with no evidence of a smear result? (How many pulmonary cases were registered as clinical cases with no bacteriological evidence of TB?) 2.4 New adult smear positive cases How many smear positive cases over the age of 15 were registered during in the last quarter? 2.5 Retreatment cases How many retreatment cases were registered during the last quarter? 2.6 New extrapulmonary TB cases How many new extrapulmonary TB cases were registered during in the last quarter? Facility level data collection form
Routinely reported program outcomes – Smear conversion and treatment outcomes Use the most recent quarter for which both smear conversion and treatment outcomes on smear-positive pulmonary TB cases are complete.
Quarter 1 (January-March) 2 (April-June) 3 (July-September) 4 (October-December)
Year ______Total number of new smear positive patients registered in this cohort ______
Total number of retreatment cases registered in this cohort ______
Indicator Data needs Value Data Source 2.7 New cases with no smear conversion result How many cases in this cohort were tested for smear conversion after the intensive phase of treatment? 2.8 Sputum-conversion rate at the end of the How many cases in this cohort were smear initial phase of treatment negative at the end of the intensive phase of treatment? 2.9 Cure rate How many cases in this cohort were smear negative at the end of the treatment regimen? 2.10 Treatment completion rate How many cases in this cohort completed treatment but do not meet the criteria for cure or failure? 2.11 Death rate How many cases in this cohort died during treatment (of any cause)? 2.12 Treatment failure rate How many cases were smear positive any time after 5 months of treatment? 2.13 Default rate How many cases interrupted treatment for more than 2 months? 2.14 Transfer out rate How many cases transferred to another facility or district and do not have any treatment outcome reported from that location? 2.15 Retreatment failure rate How many retreatment cases registered during this same period were smear positive after taking a retreatment regimen? Facility level data collection form
DOTS implementation indicators
Indicator Data needs Value Data Source 3.3 National TB Program manual Does the clinic have a copy of the most recent NTP manual or Yes/No DOTS guidelines? 3.9 Key NTP staff positions filled How many people are working full time in the DOTS program Yes/No at this clinic? Are these positions filled by local employees? Yes/No
4.1 Existence of a comprehensive laboratory What is the reference laboratory for this clinic? (Where do network you send sputum for culture and/or drug susceptibility testing?) 4.2 TB microscopy coverage What is the catchment area for microscopy services? (This may be larger than the catchment area for the general services since microscopy is not always available.) 4.3 TB microscopy units with adequate How many slides per day are prepared and read by this workloads laboratory? How many full-time technicians are working in the laboratory? 4.4 TB microscopy units submitting slides for Does this laboratory routinely submit slides to the reference Yes/No rechecking laboratory for rechecking? (Ask to see the most recent QA report.) If so, how often does the rechecking occur? 4.5 TB suspects who are smear positive How many TB suspects were found to be smear positive during the last quarter? How many TB suspects were identified clinically during the last quarter? 4.6 Smear-negative TB cases properly How many smear-negative TB cases were reported by this diagnosed facility in the past quarter? How many smear-negative TB cases have at least 3 negative sputum tests recorded? How many smear-negative TB cases have chest X-ray results recorded? 4.7 Detected smear-positive cases registered How many smear-positive cases that were detected in the last for treatment (Inverse of primary default rate) quarter began treatment? What is the total number of smear positive cases that were Indicator Data needs Value Data Source detected during the last quarter? 5.1 Patients under direct observation of How many new smear-positive TB cases reported having therapy every dose of medication observed per NTP guidelines? *If interviews are not possible, this question How many new smear-positive TB cases were interviewed can be answered by looking over individual regarding direct observation of therapy? treatment cards to see if doses are recorded as directly observed. 5.2 New TB patients who were prescribed the How many TB patients completed treatment during the last correct regimen quarter? How many of these patients were prescribed the correct regimen of anti-TB drugs during the last quarter? 6.7 Basic management units where anti-TB Are all of the first-line anti-TB drugs used by the NTP Yes/No drugs are available available in this clinic? 6.8 Anti-TB drug samples that fail quality Does this clinic participate in quality-control activities Yes/No control tests implemented by the NTP? For example, does the NTP routinely take samples of drugs from the facility and test them? 7.1 Completeness of reporting to the NTP Were the most recent case detection and treatment outcome Yes/No *Ask to see a copy of the most recent case reports submitted on time to the NTP? detection/treatment outcome reports submitted Was all of the required information entered for these reports? Yes/No to the NTP. 7.2 Accuracy of reporting to the NTP Using the same report, determine whether or not it was filled Yes/No out correctly. 8.1 Supervision of DOTS implementation When did this clinic last receive a supervisory visit? Is the supervisor’s report available? Yes/No 9.1 TB microscopy units with at least one If microscopy services are available, is there at least one Yes/No technician trained in AFB microscopy within technician who was trained in AFB microscopy during the past the past 3 years year? Is his/her certificate on file at the facility? Yes/No 9.2 Health care units with at least one health How many health care workers in this facility have been care professional trained in TB-case detection trained in DOTS-based case detection and treatment within the and treatment past three years?