Tutorial: Blackbody Radiation (Adapted from Lecture-Tutorials for Introductory Astronomy, CAPER Team, Preliminary Edition, 2002)
Part I: Spectral Curves
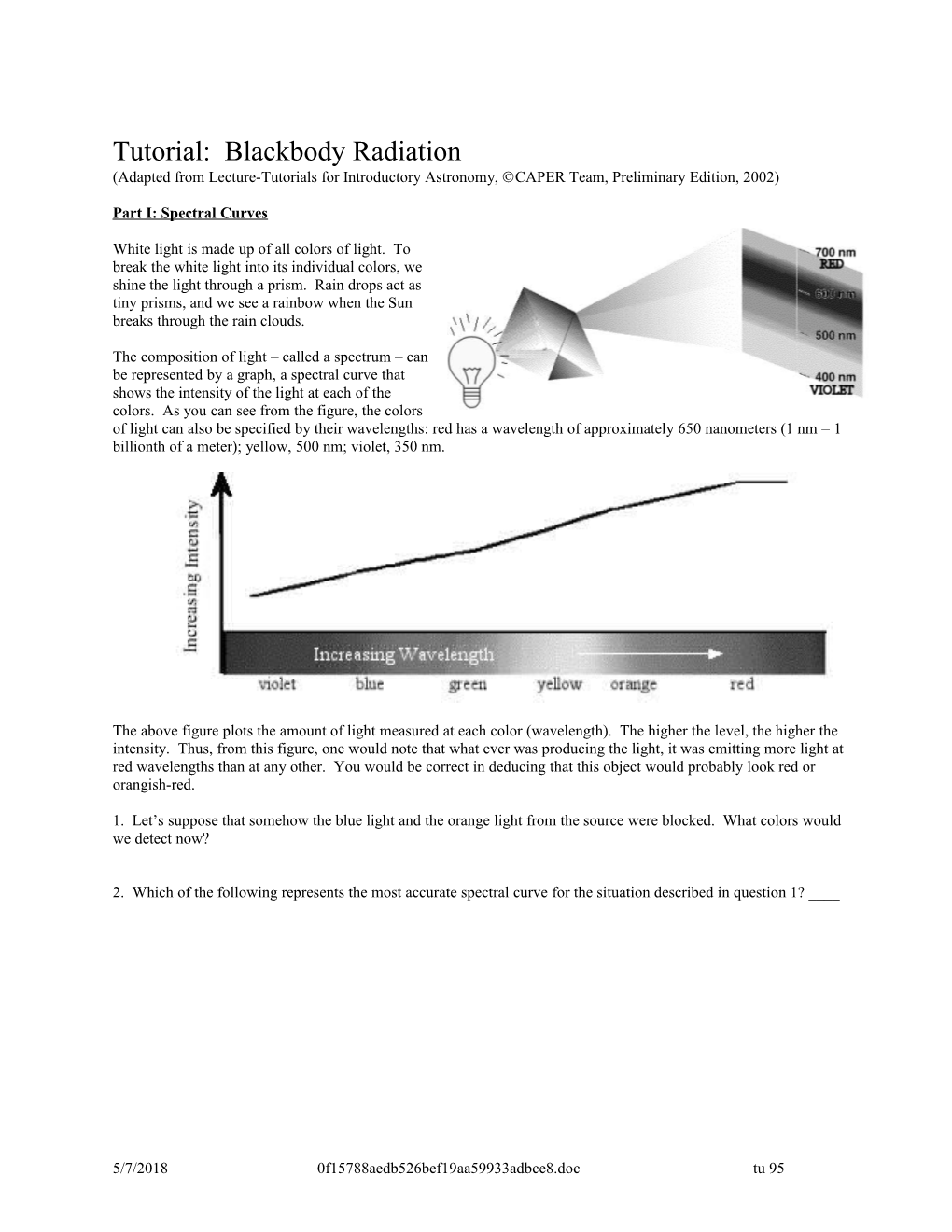
White light is made up of all colors of light. To break the white light into its individual colors, we shine the light through a prism. Rain drops act as tiny prisms, and we see a rainbow when the Sun breaks through the rain clouds.
The composition of light – called a spectrum – can be represented by a graph, a spectral curve that shows the intensity of the light at each of the colors. As you can see from the figure, the colors of light can also be specified by their wavelengths: red has a wavelength of approximately 650 nanometers (1 nm = 1 billionth of a meter); yellow, 500 nm; violet, 350 nm.
The above figure plots the amount of light measured at each color (wavelength). The higher the level, the higher the intensity. Thus, from this figure, one would note that what ever was producing the light, it was emitting more light at red wavelengths than at any other. You would be correct in deducing that this object would probably look red or orangish-red.
1. Let’s suppose that somehow the blue light and the orange light from the source were blocked. What colors would we detect now?
2. Which of the following represents the most accurate spectral curve for the situation described in question 1? ____
5/7/2018 0f15788aedb526bef19aa59933adbce8.doc tu 95 tu 96 3. What colors of light are most intense in 3 b) above?
4. What colors are present in 3 c) above? Would the object producing this light appear bluish or reddish?
Part II: Blackbody Curves (Wien’s Law)
Different colors of light are manifestations of the same phenomenon but have different 2.9106 wavelengths. For example, red light has a wavelength between 650 and 750 nm, while nm violet/blue light has a wavelength between 350 and 450 nm. Stars also radiate light at peak Temp (K) wavelengths outside the visible range – at ultraviolet and infrared wavelengths.
The two most important features of a star’s blackbody radiation curve are its maximum height—an indication of the star’s energy output—and the wavelength at which this occurs—called the peak wavelength. For example, if Star A and Star B are the same size and temperature, they will have identical blackbody curves. If Star C is the same size as A and B but is cooler, its energy output is reduced at all wavelengths and the peak of the curve occurs at a longer wavelength.
1. Which spectral curve shows more red light?
2. Which spectral curve shows more blue light?
3. Which star, A or C, will look redder? ______What was the logic you used in answering this question?
4. Two students are discussing their answer to question 3: ABE: Star A looks redder because it is giving off more red light than Star C. BABS: Abe, you are ignoring how much blue light Star A gives off. Star A gives off more blue light than red light, so it must look bluish. Star C has more red that blue, so it looks reddish. Star C is redder than Star A.
Do you agree with Abe or Babs, or neither one? Explain your answer.
5. Star A Star C Star D All None Shortest peak wavelength Lowest surface temperature Greatest energy output Would look the brightest at IR wavelengths
6. How must Stars C and D be different from Stars A and B to account for the difference in energy output?
7. Let’s say you measured the blackbody spectrum of Star E and found that the peak intensity was just as great as star A, but the peak wavelength was at 750 nm rather than 600 nm. What can you state given this information? a. Star E is smaller and cooler than Star A. b. Star E is hotter and larger than Star A. c. Star E is cooler but must be larger in size than Star A. d. This situation is physically impossible.
97 e. There isn’t enough information to come to any conclusions given this information.
tu 98