BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Lecture #9 – Cardiovascular Modeling
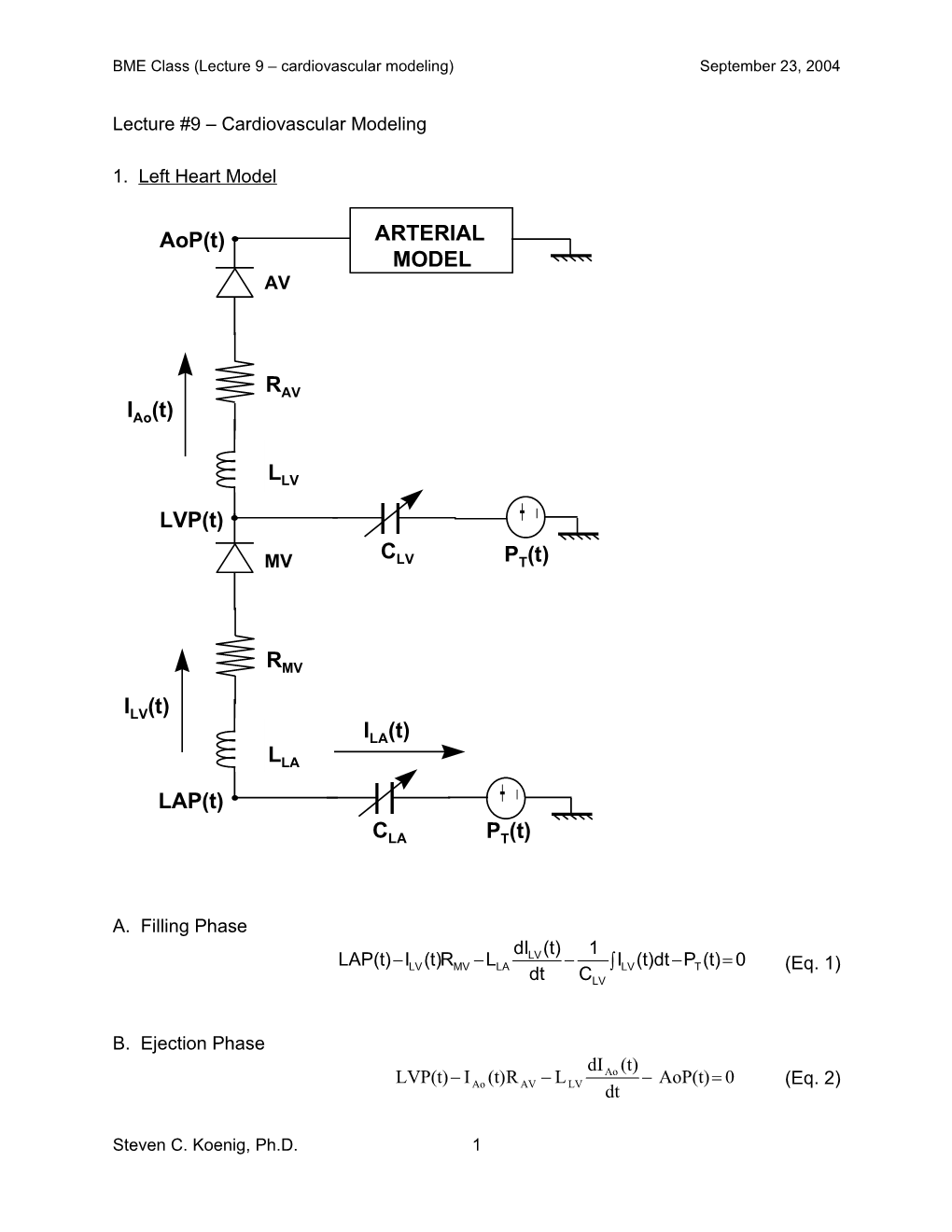
1. Left Heart Model
AoP(t) ARTERIAL MODEL AV
RAV IAo(t)
LLV - LVP(t) + C MV LV PT(t)
RMV
ILV(t) ILA(t) LLA - LAP(t) +
CLA PT(t)
A. Filling Phase
dILV (t) 1 LAP(t)ILV (t)RMV LLA ILV (t)dt PT (t) 0 (Eq. 1) dt CLV
B. Ejection Phase dI (t) LVP(t) I (t)R L Ao AoP(t) 0 (Eq. 2) Ao AV LV dt
Steven C. Koenig, Ph.D. 1 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
C. Parameter Estimation - integral approach (ejection phase)
t1 t1 [LVP(t) AoP(t)]dt - R I (t)dt L [I(t ) I(t )] 0 AV Ao LV 1 0 (Eq. 3a) t0 t0
t2 t2 [LVP(t) AoP(t)]dt - R I (t)dt L [I(t ) I(t )] 0 AV Ao LV 2 1 (Eq. 3b) t1 t1
2. Background: ‘old’ modeling concepts Introduction to Myocardial Mechanical Properties: Myocardial visco-elastic properties can be described by two parameters: elastance and resistance. Elastance is defined as the ratio of the pressure response to changes in volume. The time-varying theory of elastance was constructed to explain the pressure volume behavior of the contracting heart (Robinson 1965, Suga 1969). The concept of viscous losses (friction) within the shortening myocardium is commonly referred to as resistance and has been quantified by numerous investigators, and is a critical element in the Hill relationship (Sonnenblick 1962a and 1962b).
Current Elastance-based Methods of Evaluating Cardiac Performance: For years, it was thought that the end-systolic-pressure-volume-relationship (ESPVR) and other elastance-based methods were indicative of cardiac performance (Sunagawa 1980, Takeuchi 1991, Shih 1997, Senzaki 1996, Shishido 2000). Recently, however, more investigators have shown that ESPVR is not as predictive of cardiac contractility as once thought. In an excellent review of four elastance-based techniques, Kjorstad et al (2002) concluded that, “It is therefore doubtful whether any of the methods allow for single-beat assessment of contractility.”
Introduction to Elastic Pressure and Source Resistance Concepts: Others (Campbell 1990, 1997, Hunter 1980,1983, Chang 1997) have unified the elastance and resistance concepts in an elastance-resistance (E-R) cardiac model and perhaps the most intuitive E-R model to understand is a mechanical model, as shown in Figure 9.1. Here the time varying elastance is denoted by a variable spring element and the viscous (frictional) loss is represented by a dashpot element. As the elastance becomes greater, the spring produces a greater force and pushes upward producing a force (pressure) of PELAST. When the left ventricular pressure becomes greater than the aortic pressure, the aortic valve opens and volume is ejected as the piston moves upward. As it moves upward, there is a resisting force (pressure) due to the dashpot (PDASHPOT) that reduces the net force upward (PELAST - PDASHPOT) and it is balanced by LVP. Thus whenever there is motion as in ejection (or filling), the pressure due to the elastic (force-producing) element, PELAST, is no longer equal to the left ventricular pressure (Figure 9.2).
Steven C. Koenig, Ph.D. 2 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Aortic Reviewer's Note: This model is an Flow intuitive abstraction of a more Aortic complex process. However, while Valve Mitral the exact details of elastance- Valve resistance models may vary, the Left mathematical findings are valid for Ventricle more complex processes. Atrial Inflow dVol LVP Elastance generates P and is elasT opposed by friction, P and dt DASHPOTP P ventricular pressure, LVP. ELAST DASHPOT
Time varying Frictional Element (Dashpot) elastance R S
Figure 9.1 Simple Elastance and Resistance Model
LVP
P P ELAST DASHPOT
Figure 9.2 Pressure Balance Diagram
The mathematical relationships between the pressures are given in equations 9.1-9.3. Note that the time rate of volume change is the negative of the aortic flow (AoF) during ejection.
LVP PELAST PDASHPOT Equation 9.1 dVol LVP P R Equation 9.2 ELAST S dt
LVP PELAST RS * AoF Equation 9.3 A major consequence of equations 9.1-9.3 is that LVP can be measured using a traditional pressure catheter, but neither PELAST or PDASHPOT can be directly measured. PELAST can only be indirectly estimated whenever PDASHPOT = 0 as this is when LVP = PELAST (i.e. during an isovolumic phase, Equation 1). Finally, traditional E-R models have fallen short of predicting cardiac performance, particularly in the late systole and
Steven C. Koenig, Ph.D. 3 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004 diastolic relaxation phases, leading some researchers to suggest that E-R approaches may not adequately describe this sub-process (Campbell 1990). A significant aspect of using PELAST and RS is that volume need not be measured and Vo (the ventricular filling volume) need not be determined using a vena caval occlusion, making this approach more clinically suitable.
Previous Approaches to Estimating PELAST. Sunagawa assumed that the isovolumic contraction and relaxation phases of an ejecting beat could be used to predict the pressure waveform of an isovolumic beat. He used an inverted cosine function and adjusted its amplitude, PMAX, its duration, T and its phase term, , until both the isovolumic contraction and relaxation phases of an ejecting beat each lay along opposite tails of the inverted cosine curve. The portion of the inverted cosine curve between the two isovolumic phases described the pressure the beat would have attained had it not ejected. In other words, the inverted cosine curve described PELAST of an isovolumic beat. Two significant problems can arise when using this approach. First, the duration of an isovolumic beat is longer than an ejecting beat, when both start at the same ventricular volume. Sunagawa's approach assumed the isovolumic and ejecting waveforms had equal duration, T. Second, the cosine basis function requires that the isovolumic contraction and relaxation phases to be mirror images in the sense that they each would both lie along the respective tails of an inverted cosine curve, which is often not the case.
In fact, the ejecting beat diastolic relaxation dynamics are often different than the isovolumic contraction dynamics, prompting some investigators to model the two isovolumic phases of an ejecting beat with different exponential-form equations with different time constants (Regen 1994). It is very important to note that Sunagawa’s PMAX estimate is fundamentally different that our PELAST estimate. Sunagawa’s PMAX was considered to be the isovolumic pressure, while our PELAST is the pressure that results from a lossless ventricle during an ejection process. PELAST and PMAX are equivalent only when the beat is completely isovolumic.
Determining PELAST and RS during Ejection: Any shortening of the cardiac muscle (i.e. ejection) results in less generated LVP, as energy that could have been used to develop LVP is lost in the myocardial friction or resistance, RS, (PELAST - PDASHPOT = LVP).
Since there is no aortic flow during the contraction and relaxation regions of a beat there are no losses due to RS and therefore PELAST is equal to LVP in these regions. PELAST is not known during the ejection portion of a beat because aortic flow is not zero and a pressure loss occurs due to resistance, RS. In order to determine PELAST during ejection a method was developed using a cubic spline interpolation to predict what the pressure would have been over this region similar to the approach taken by Kjorstad (2002). Briefly, the beginning and end of ejection was selected using dpdt maximum and minimum respectively. These points correspond to the end of contraction and beginning of relaxation. Next, a threshold on LVP dp/dt was employed to determine the beginning of contraction and the end of the relaxation region. With these four points selected, the analysis region of the ejecting beat was established. PELAST from the
Steven C. Koenig, Ph.D. 4 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004 isovolumic regions was used as input data in a cubic spline interpolation to predict PELAST during ejection. Once PELAST is known RS was determined using the following formula. P LVP R ELAST S AoF The programs for this procedure were developed and implemented in Matlab. The application of this technique to normal and failing hearts is described below.
Canine Heart Failure PELAST and RS (Data Courtesy of L. Mulligan, Medtronic Heart Failure Group): A rapid pacing model in a canine was used to create heart failure. Data were obtained in normal and heart failure models and the results of the preliminary study are depicted in the following three figures.
Clearly, and not surprisingly, PELAST is depressed in heart failure indicating that the pressure generation component has been affected and its isovolumic relaxation phase is prolonged, which may be partly explained by the increase in RS in heart failure. Overall RS is increased for all values of PELAST. An increase in RS may increase the time constant of relaxation, resulting in a sluggish isovolumic relaxation phase. Also, RS has a unique behavior in heart failure as it starts out very high and then drops as PELAST increases. Next as PELAST decreases in the latter part of ejection, RS drops in proportion. In fact this early elevated RS may be indicative of a static friction (“stiction”) phenomenon. In stiction, the sliding elements are “sticky” at first, then as motion begins they become “unstuck” resulting in less friction. The response of heart failure Panel A Panel B
Figure 9.3 PELAST waveforms (Panel A) for control and heart failure, (Panel B) RS under normal and heart failure conditions. In (Panel B) the circles in the lower left indicate the end of ejection.
RS in early ejection decreases with increasing Pelast and opposite to that observed by Hunter (1979) in isolated hearts. RS seen in the normal condition increase with Pelast and agrees with experimental observations of Hunter.
Steven C. Koenig, Ph.D. 5 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Preliminary Swine PELAST and RS: A Yorkshire pig was used as the animal model to obtain ejecting hemodynamic data to estimate both PELAST and RS. Shown below in Figure 9.4 are representative plots of PELAST for a normal porcine heart.
One can see that for a healthy swine heart, PELAST exceeds LVP significantly during ejection and matches the isovolumic phases exactly. The reduction of PELAST to LVP during ejection is due to the internal frictional losses (RS). Overall, RS is roughly proportional to PELAST, as one expects from experimental observations (Hunter et al) and is not appreciably different at the higher heart rate. However, it does exhibit different behavior for increasing PELAST than for decreasing PELAST. The largest RS occurs at or near the largest PELAST, which coincides with the largest ventricular outflow. The result of this is that the largest frictional losses occur at the largest flow values, just when RS is at its greatest value. This demonstrates our ability to make these calculations and this data set will be used to compare to normal human hearts in assessment of hemodynamic compatibility of xenotransplantation.
Panel A Panel B
Figure 9-4. Panel A at heart rate of 90 beats per minute (bpm) and Panel B at heart rate of 130 bpm. Top Panel A and B shows PELAST estimates derived from LVP and lower panel A and B shows similar RS for a swine heart. Here the circle denotes the beginning of ejection.
Steven C. Koenig, Ph.D. 6 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Preliminary Human Heart Failure PELAST and RS: After informed consent, patients just prior to undergoing a partial left ventriculectomy were instrumented according to procedures approved by IRB. Briefly, left ventricular and aortic pressure, and aortic flow were recorded for a two-minute period under steady state conditions. PELAST and RS were estimated from the recorded waveforms using the new technique described above (AHA grant 0051419Z). The figures below show the results of one test patient. This demonstrates that we have the expertise to make calculations in human subjects in a clinical setting. Further, this patient set will be analyzed and the results compared to normal human hearts (pre-CABG) to establish baseline differences in normal and failing human hearts.
In the Panel A of Figure 9.5, it is evident that PELAST is not very much higher than LVP during ejection. Overall the LVP diastolic pressures are very high and the systolic pressures are very low. Interestingly, RS, displays the same behavior as the failing dog heart – that is it starts out elevated and drops as PELAST increases to its maximum value, then as PELAST falls, so does RS (as shown in Figure 9.6).
Figure 9.5 Human heart in heart failure with showing small PELAST as compared to LVP. Right panel shows RS vs. PELAST and “stiction” phenomenon. Here the circle denotes the beginning of ejection.
Steven C. Koenig, Ph.D. 7 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Figure 9.6 Illustration of similarity of shape of source resistance vs PELAST for heart failure in canine and humans.
Summary. It is believed that cardiac function can be cleaved into two components – a pressure generating function (PELAST) and the ability to empty volume effectively (RS). We have shown that clear differences in PELAST and RS exist between normal and failing canine hearts, where PELAST is depressed and RS is elevated in heart failure. In fact RS is very high at the start of ejection for both failing canine and human hearts, perhaps indicating a type of ‘stiction” (static friction). In heart failure, PELAST was depressed indicating an inability of the heart to generate adequate pressure, concurrently, RS is elevated making the ejection process less effective. Thus in these failing hearts both components have been adversely affected.
Ultimately, cleavage of cardiac function into these two components -- pressure generation (PELAST) and effective ejection(RS) may offer a better way to assess various treatment options. For example, PELAST might be improved with treatment A and RS might be improved with treatment B and a combined therapy improves both. Importantly, PELAST and RS may provide metrics to analyze the mechanisms (genetic and metabolic) responsible for changes in pressure generation and volume ejection.
Steven C. Koenig, Ph.D. 8 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Lumped Parameter Vascular Models:
C R
(a)
Zo L
C R C R
(b) (c) R’ L LP
C R C R Zo
(d) (e) Figure 9.1 Assorted lumped parameter models of systemic vasculature: (a) 2- element Windkessel, (b) 3-element Winkessel, (c) 3-element inductance, (d) 4-element Noordergraf, and (e) 4-element Windkessel or Buratini model.
A. Time Domain Analysis (2-element Windkessel model (a))
The model equations for estimating the parameters are defined by, t1 t1 1 t1 I dt C dAoP(t)dt AoP(t) - RAP(t)dt (Eq. 9.1a) Ao R t0 t0 t0 t2 t2 1 t2 I dt C dAoP(t)dt AoP(t) - RAP(t)dt (Eq. 9.1b) Ao R t1 t1 t1 where, the aortic flow (IAo), aortic pressure (AoP(t)), and right atrial pressure (RAP(t)) can be measured. Using this 2 equations and 2 unknowns approach, we can solve for R and C by: Area(IAo) = C[(AoP(t1) – AoP(t2)] + 1/R[Area(AoP – RAP)]
B. Spectral Analysis (3-element Inductance model (c))
Steven C. Koenig, Ph.D. 9 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Theory – The impedance equations for the arterial models (b-e) can be derived by taking the Laplace Transform of the model equations. Define P(t) = aortic pressure and Q(t) = aortic flow.
The transformed arterial parameters and measurements are defined as,
Time Transformation Domain (S-Domain) R R C 1/(sC) L sL P(t) P(s) Q(t) Q(s)
In the case of the 3 element inductance model (c), the impedance equation is derived as follows: 1 R P(s) sC T(s) sL (Eq. 9.2) Q(s) 1 R sC 1 R P(s) sC Cs R T(s) sL sL (Eq. 9.3) Q(s) 1 Cs (RC)s 1 R sC P(s) R (RC)s 1 (RC)s 1(sL) R T(s) sL (Eq. 9.4) Q(s) (RC)s 1 (RC)s 1 (RC)s 1
s2 (LCR) s(L)R T(s) (Eq. 9.5) (CR)s 1 and, evaluating the impedance equation at s = j = j2f, R j (LC2R2 )3 (L CR2 ) T(j) (Eq. 9.6) (C2R2 )2 1 The impedance equation evaluated at s = j (Eq. 9.6) can then be broken into real and imaginary components (Eqs. 9.7a and 9.7b). A R Re(j) Re (Eq. 9.7a) D (C2R2 )2 1 2 2 3 2 A (LC R ) (L CR ) Im(j) Im (Eq. 9.7b) D (C2R2 )2 1
The magnitude and phase at each frequency (f1, f2, … fn) can be calculated:
Steven C. Koenig, Ph.D. 10 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Magnitude = Re(jω)2 Im(jω)2 (Eq. 9.8a)
1 Im(jω) Phase = tan (Eq. 9.8b) Re(jω) Using Eq. 9.8a and 9.8b, we can then calculate the magnitude and phase for n frequency terms.
Now, to estimate L-R-C paramters, … Look at magnitude and phase information by applying the Fast Fourier Transform (FFT) to the measured aortic pressure, P(t), and aortic flow, Q(t). We can then adjust the R-L-C parameters until a ‘good fit’ between the measured and calculated magnitude(s) and phase(s) for n frequency terms is achieved.
Parameter Estimation Technique We can estimate the R-L-C parameters in Matlab with the ‘fminsearch’ function.
FILENAME: 3element_Lmodel.m global Zexp nharm w Zmod AoF=AoF1*1000/60; % convert from L/min to cc/sec fs=400; % sampling frequency (Hz) Zexp=fft(AoP1)./fft(AoF); % experimental input impedance nharm=3; % number of harmonics w=fs/length(AoP1); % fundamental frequency (Hz) XX=fminsearch('Z_freq', [1,1]) % call fminsearch and initial conditions [C L] FILENAME: Z_freq.m function error=Z_freq(pp) % Function minimizes error between experimental and model impedances % by iteratively adjusting L and C global nharm w Zexp Zmod harm=[1:nharm];
% Calculate impedance for model for 'nharm' harmonics for 3L model for ii=1:nharm-1 Z1(ii)=-j/(ii*w*pp(1)); % impedance of capacitor Z2(ii)=Zexp(1); % impedance of resistor Z3(ii)=j*ii*w*pp(2); % impedance of inductor Zp(ii)=Z1(ii)*Z2(ii)./(Z1(ii)+Z2(ii));% impedance of R-C parallel Zmod(ii)=Zp(ii)+Z3(ii); % impedance of Zp + L series
real_e(ii)=(ii.^2)*(real(Zexp(ii+1))-real(Zmod(ii))); % weighting imag_e(ii)=(ii.^2)*(imag(Zexp(ii+1))-imag(Zmod(ii))); % weighting end error=sum(real_e.^2)+sum(imag_e.^2); AA=abs(Zmod); BB=abs(Zexp); plot(harm,[Zexp(1) AA(1:nharm-1)],'*',harm,BB(1:nharm),'.') pause(0.1)
Steven C. Koenig, Ph.D. 11 BME Class (Lecture 9 – cardiovascular modeling) September 23, 2004
Assignment #4 1. Derive the impedance equations for the 4-element Windessel model (e).
2. Using ‘patient001’ and ‘patient002’, data files and approach discussed in lecture modify sample code, and estimate R and C parameters for the 4-element Windkessel model (a) for the first 3 beats of data. Compare parameter values for each data set. Are they different? If so, why do you think they are different (i.e. what is the condition of the ‘patient’ in each of these data sets (normal, failure, or recovery)?
Note: Data were sampled at 400 Hz (or 1 sample every 2.5 msec). AoP = aortic pressure (mmHg) AoF = aortic flow (L/min)
*Assignment #9 - due in class on Tuesday, September 30, 2004)
Reference: 1. Essler S, MJ Schroeder, V Phaniraj, SC Koenig, RD Latham, and DL Ewert. Fast estimation of arterial vascular parameters for transient and steady beats with application to hemodynamic state under variant gravitational conditions. Ann. Biomed. Eng., 27:486-97, July 1999.
Steven C. Koenig, Ph.D. 12