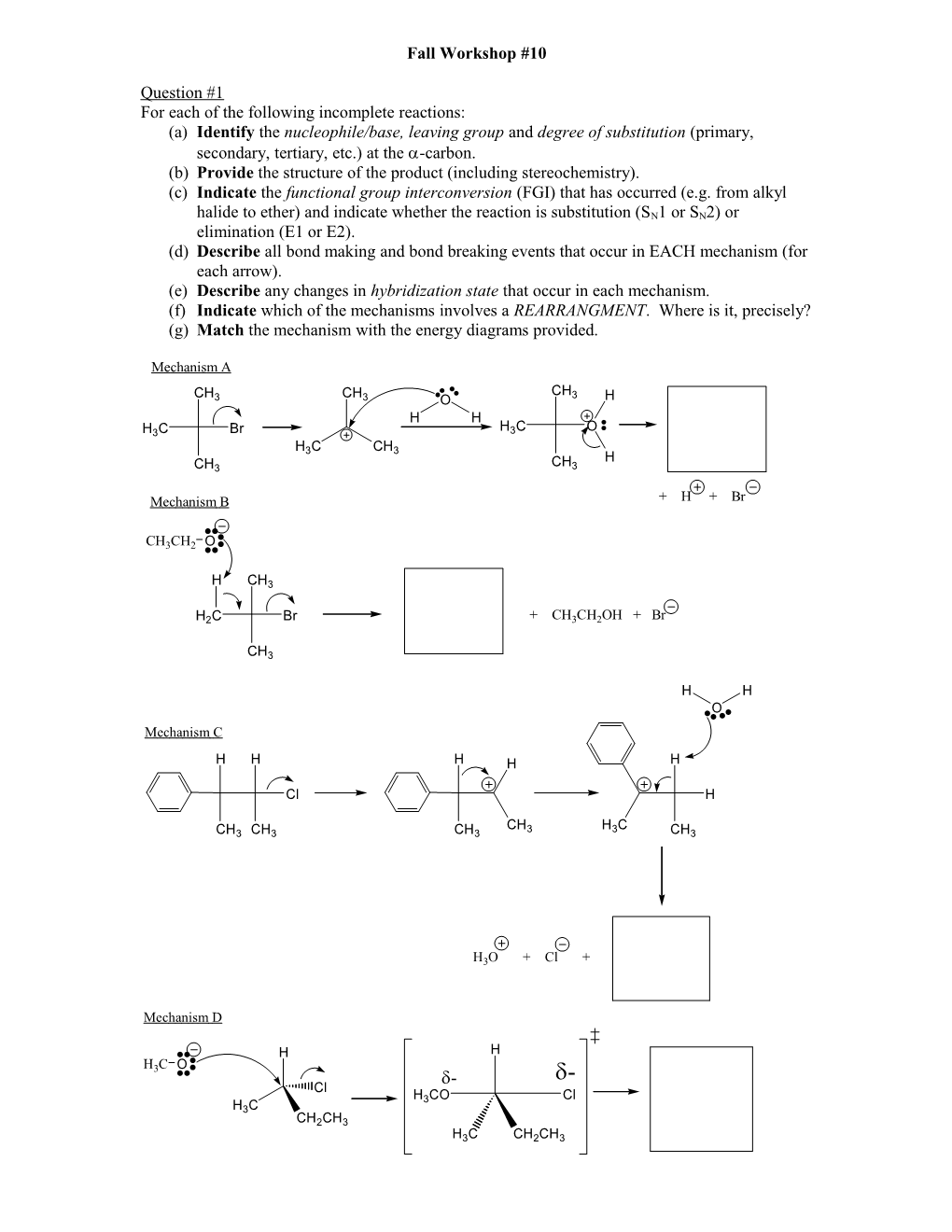
Fall Workshop #10
Question #1 For each of the following incomplete reactions: (a) Identify the nucleophile/base, leaving group and degree of substitution (primary, secondary, tertiary, etc.) at the -carbon. (b) Provide the structure of the product (including stereochemistry). (c) Indicate the functional group interconversion (FGI) that has occurred (e.g. from alkyl
halide to ether) and indicate whether the reaction is substitution (SN1 or SN2) or elimination (E1 or E2). (d) Describe all bond making and bond breaking events that occur in EACH mechanism (for each arrow). (e) Describe any changes in hybridization state that occur in each mechanism. (f) Indicate which of the mechanisms involves a REARRANGMENT. Where is it, precisely? (g) Match the mechanism with the energy diagrams provided.
Mechanism A
CH CH CH3 3 3 O H H H H3C Br H3C O H C CH 3 3 H CH3 CH3
Mechanism B + H + Br
CH3CH2 O
H CH3
H2C Br + CH3CH2OH + Br
CH3
H H O Mechanism C
H H H H H
Cl H
CH H C CH3 CH3 CH3 3 3 CH3
H3O + Cl +
Mechanism D
H H H3C O - - Cl H3CO Cl H3C CH2CH3 H3C CH2CH3 TS1 TS2
E I1
TS3
P R I2 TS1
E Rxn Coordinate
R TS P 1 TS2
Rxn Coordinate I1 TS3 E
I2
R P
Rxn Coordinate
Question #2 In each of the following, three organic halides are shown. Indicate the order of reactivity in each designated grouping (1 is most reactive & 3 is least). Be sure to describe WHY you ranked them as you did – e.g. identify the major factor/influence that determines the rank ordering. Note that
C6H5- represents a phenyl group.
(A) SN2 substitution by NaOCOCH3 (sodium acetate) in DMF:
CH3CH2CH2Br (CH3)2CHBr CH2=CHCH2Br
(B) SN1 substitution by H2O (solvolysis):
C6H5Cl C6H5CH2Cl C6H5CHClCH3
(C) SN2 substitution by NaCN in acetone:
CH3CH2Cl CH3CH2F CH3CH2I
(D) SN2 substitution by NaSCH3 in DMSO:
(CH3)3CCH2Br CH3)2CHCH2CH2Br CH3CH2CHBrCH2CH3
(E) SN1 substitution by NaBr in methanol:
CH3Cl (CH3)3CCl CH3CH2CHClCH3
Question #3 Given the following incomplete reaction:
Cl
CH 3 CH3CH2OH
CH3
(a) Name the skeletal structure for the reactant in the reaction above. (b) Draw a CIRCLE around the nucleophile in the reaction. (c) Draw a SQUARE around the best leaving group in the reaction. (d) Place a CHECK MARK next to the any electrophilic carbons. (e) Is the leaving group attached to a primary, secondary, or tertiary carbon? (f) Do you have a good leaving group or poor leaving group? (g) Is your nucleophile a strong base or weak base? (h) Do you have a good nucleophile or a poor nucleophile? (i) What type of solvent is used in this reaction – PAD or PPD (See SAM pg. 10)? Why do we want to use this type of solvent in this case?
(j) Given your answers to parts (e-i), do you think this reaction is more likely SN1/E1, SN2 or E2? (k) Provide a mechanism showing all relevant transition states/intermediates for the reaction chosen. (l) Provide skeletal structure(s) of the expected MAJOR product(s) ONLY in the box provided (above). Name your products. (m) Provide the rate law equation for this reaction. (n) How would the reaction change if you made the following alterations to the reaction conditions?
1. you change the nucleophile to sodium ethoxide and the solvent to ethanol 2. you change the nucleophile to sodium acetate and the solvent to DMF 3. you change the leaving group to a tosylate 4. you change the leaving group to NH2 5. you double the concentration of R-X 6. you quadruple the amount of ethanol 7. you change the starting R-X to:
H Cl H
CH3 Question #4 (a) Even though the reaction shown below can potentially give two stereoisomeric elimination products, only one is formed. Give the structure for this single alkene product. HINT: You’ll need to build a molecular model using your model kit!
(b) Either of the two diastereomers of the tosylate shown in part (a) can also react with potassium t-butoxide to give an alkene (HINT: again, use your model kit). This alkene is different than the alkene product from part (a). Provide the structure of this alkene product. Clearly account for the stereochemical course of these two reactions. Why is there no cross- over? Explain in detail using your model and write out a reaction mechanism. Also, account for why tosylates are such good leaving groups.
(c) Given what you learned in parts (a) and (b), try to predict the products for the following 4 reactions:
I NaOH Ethanol
CH3
I NaOH Ethanol
CH3