Review of arrangements for scheduling substances under Part 6-3 of the Therapeutic Goods Act 1989 - summary of issues raised in written submissions, public forums and meetings and how the panel dealt with these issues
System of access controls Section 52E Secretary to take certain matters into account
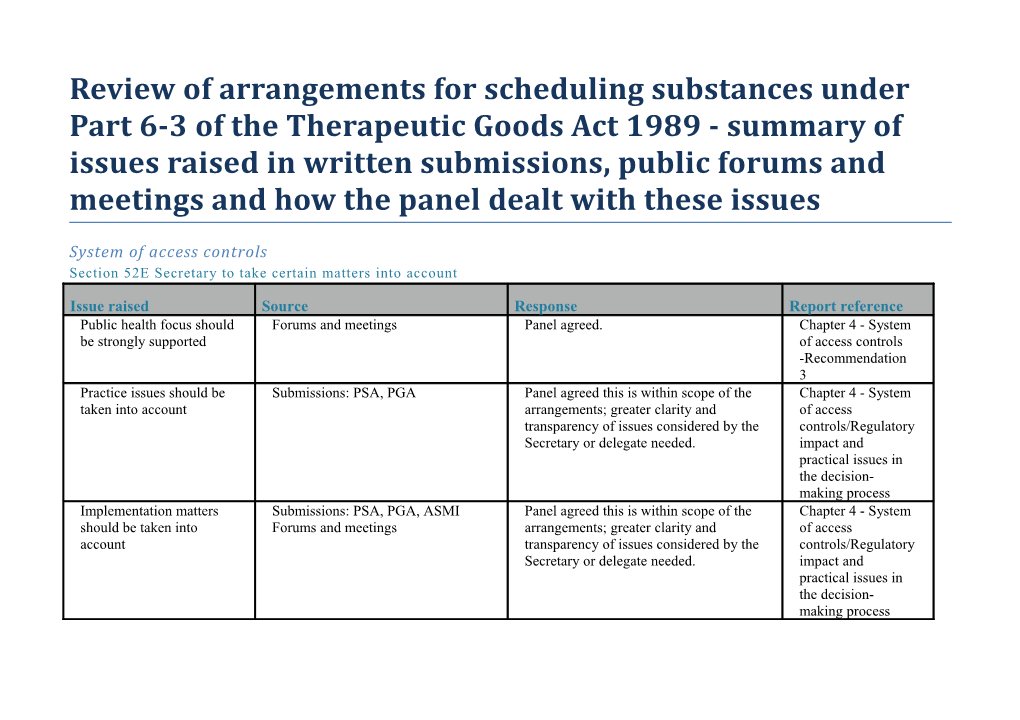
Issue raised Source Response Report reference Public health focus should Forums and meetings Panel agreed. Chapter 4 - System be strongly supported of access controls -Recommendation 3 Practice issues should be Submissions: PSA, PGA Panel agreed this is within scope of the Chapter 4 - System taken into account arrangements; greater clarity and of access transparency of issues considered by the controls/Regulatory Secretary or delegate needed. impact and practical issues in the decision- making process Implementation matters Submissions: PSA, PGA, ASMI Panel agreed this is within scope of the Chapter 4 - System should be taken into Forums and meetings arrangements; greater clarity and of access account transparency of issues considered by the controls/Regulatory Secretary or delegate needed. impact and practical issues in the decision- making process Issue raised Source Response Report reference Cost-benefit and/or Submissions: Accord, ASMI, PSA Panel noted OBPR and regulatory impact Chapter 4 - System regulatory impact Forums and meetings assessments are within scope of the of access assessment should be arrangements. controls/Regulatory taken into account impact and practical issues in the decision- making process Information considered by Submissions: ADA, PSA, PGA Panel agreed that transparency is Chapter 4 - Clarity the advisory committees Forums and meetings important but needs to be balanced and transparency of and delegate should be against protecting personal, professional process to amend public and commercial business information. the Poisons Standard Circumstances when Submissions: PSA, Accord, CHF Panel agreed that more information Chapter 4 - System Secretary amends Poisons Forums and meetings could be made available. of access Standard on own initiative controls/Clarity and should be clear transparency of process to amend the Poisons Standard How the delegate Submissions: PGA, Accord, ASMI Panel noted that secretariat and delegates Chapter 4 - System exercises his/her power Forums and meetings are considering the level of detail to be of access How risk/benefit and risk included in a notice of decision. controls/Clarity and management decisions are Panel recommended that level of detail, transparency of made clarity and transparency contained in process to amend How the advisory public notices is improved the Poisons committees and delegate (Recommendation 1). Standard applied the SPF Issue raised Source Response Report reference Policy oversight, Submissions: PSA, PGA, ASMI Panel recommended establishment of a Chapter 4 - System development and Forums and meetings mechanisms for policy oversight of access maintenance of the (Recommendation 2). controls/Scheduling Scheduling Policy Policy Framework Framework (SPF) and for Medicines and other guidelines relevant Chemicals/ Policy under s52E oversight review and development
Implications arising out of Submissions: Accord, ASMI Panel understands that implications for Chapter 8 - Other the proposed Forums and meetings scheduling arrangements will be matters/ Transition establishment of a trans- addressed. to the Australia Tasman joint medicines New Zealand agency Therapeutic Products Agency Need for more guidance Submissions: PSA, PGA, ASMI, Panel recommended that certain policy Chapter 4 - System on the: APVMA issues be considered (Recommendation of access -matters the Secretary or Forums and meetings 3). controls/Scheduling delegate considers Policy Framework necessary to protect public for Medicines and health Chemicals/ More -approach for risk-benefit guidance on assessment scheduling issues -principles for decision- making criteria for excluding substances -approach to combination products -data and information requirements Issue raised Source Response Report reference Streamlining processes to Submissions: Accord, ASMI, Panel recommended that certain process Chapter 4 - System enable: APVMA policy settings be considered of access -greater flexibility for Forums and meetings (Recommendation 3). controls/Scheduling delegate-only decisions Policy Framework -regulatory agency decides for Medicines and whether scheduling Chemicals/ consideration is warranted Streamlining the and/or make scheduling recommendation directly processes - ability to reject of defer applications
Better alignment with Submissions: PSA, ASMI, APVMA Panel noted effect on industry. Chapter 6 - Effects regulatory Forums and meetings The panel found that scheduling and of 2009 registration/approval regulatory processes should be aligned to amendments on process the extent possible and encouraged industry/process further work to achieve this. issues Chapter 8 - Other matters /Role, profile and integration of scheduling with other parts of regulation, policy and processes
Section 52EAA Application for amendment of the Poisons Standard Issue raised Source Response Report reference Application template: Submissions: Accord, ASMI Panel recommended application Chapter 4 - System -clarity and consistency Forums and meetings requirements be clarified and made more of access when mandated flexible (Recommendation 4). controls/Applicatio -clearer and better n for amendment of structured format needed the Poisons Standard Facilitate electronic and Submissions: CPA, Accord, ASMI, Panel noted stakeholder preference that Chapter 4 - System hard copy APVMA both electronic and hard copy formats of access are permitted. controls/Applicatio n for amendment of the Poisons Standard Outcomes of administration Section 52 A Definitions
Issue raised Source Response Report reference
Clarity and guidance for Submissions: PSA, Accord, Panel agreed that development of Chapter 5- definition of ‘substance’ APVMA guidance document could be beneficial. Outcomes of Clarity on ‘substance’ being Forums and meetings administration/Defi considered nitions
Specificity and clarity of Submissions: PSA, Accord Panel noted that Secretary or delegate Chapter 5 - schedule entries may ask advice on schedule entries. Outcomes of administration/Defi nitions
Sections 52B, Section 52C and Section 52CA Function of the ACMS and ACCS and associated Regulations Issue raised Source Response Report reference
Membership of committees: Submissions: CPA, PGA. Panel found membership categories Chapter 5- -extend range of specialist APVMA, ASMI, CMIC remain appropriate. Outcomes of expertise State and territory survey Panel encouraged Secretary or delegate administration/Func - include ACCC as observer Forums and meetings to seek specialist advice when needed. tions of the ACMS -clarify observer role Panel encouraged ACCC to be observer. and ACCS and - applicant present to Panel encouraged chairs to clarify roles associated committees of observers. Regulations/Membe Panel notes that advisory committees can rship of the call on applicant if needed. committees Greater flexibility in how and Submissions: Member of ACCS, Panel noted that ad hoc approach to Chapter 5 - when committees meet APVMA, Accord meetings may be impractical. Outcomes of Reduce joint committee Forums and meetings Panel encouraged Secretary or delegate administration/Func meetings to refine circumstances when join tions of the ACMS Implement 24 month forward meetings are required. and ACCS and meeting timetable Panel encouraged secretariat to develop a associated 24 month meeting timetable. Regulations/Admini strative arrangements of the committees
Trans-Tasman harmonisation Submissions: PGA, ASMI Panel understands that arrangements for Chapter 5 - supported Forums and meetings exchange of scheduling information Outcomes of exists. administration/Func tions of the ACMS and ACCS and associated Regulations/Commi ttee - timelines and consultation processes Issue raised Source Response Report reference
Clarify roles and Submissions: PGA, Accord, Panel recommended development of a Chapter 5 - responsibilities of the APVMA document to describe roles, Outcomes of secretariat, Secretary or Forums and meetings responsibilities and relationships administration/Func delegate and the advisory (Recommendation 6). tions of the ACMS committees and ACCS and associated Regulations/Roles and responsibilities
Administration of advisory Submissions: ASMI Panel recommended prioritisation of Chapter 5 - committees; secretariat Forums and meetings business functions and resources Outcomes of sufficiently resourced (Recommendation 5). administration/Func Panel recommended cost recovery tions of the ACMS project proceed (Recommendation 7). and ACCS and associated Regulations/Roles and responsibilities
Section 52 D Amendments to the Poisons Standard and associated Regulations Issue raised Source Response Report reference
Timelines for processes Submissions: PSA, PGA, Panel found clarity and transparency Chapter 5 - Timeliness of public Accord, ASMI, APVMA regarding timelines could be improved. Outcomes of information State and territory survey administration/Func Timeframe for implementation Forums and meetings tions of the ACMS of decisions and ACCS and associated Regulations/Commi ttee timelines and consultation processes
Adequacy of information about Submissions: PSA, PGA, Panel noted that secretariat and delegates Chapter 4 - System proposals and decisions Accord are considering the level of detail to be of access Forums and meetings included in a notice of decision. controls/Clarity and Panel recommended that level of detail, transparency of clarity and transparency contained in process to amend public notices is improved the Poisons (Recommendation 1). Standard
Increase communication of Submissions: PSA, PGA, Panel noted that TGA communication Chapter 8 - Other decisions to public Accord, ASMI and education reforms provide matters/Role, Forums and meetings opportunities for increased profile and communication. integration of scheduling with other parts of regulation, policy and processes Issue raised Source Response Report reference
Accuracy and administrative Submissions: PSA, ASMI Panel recommended prioritisation of Chapter 5 - burden of redacting business functions and resources Outcomes of (Recommendation 5). administration/Func tions of the ACMS and ACCS and associated Regulations/Roles and responsibilities
User-friendliness of website Submissions: PGS, Accord Panel noted that TGA communication Chapter 8 - Other Forums and meetings and education reforms provide matters/Role, opportunities for increased profile and communication. integration of scheduling with other parts of regulation, policy and processes
Avenues for review
Issue raised Source Response Report reference
Applicants should have Submissions: PGA, Accord, ASMI, Panel found no indication that a review Chapter 7 - Avenues access to full range of CSL Behring mechanism is required for review review processes Forums and meetings (Recommendation 8). Issue raised Source Response Report reference
Uncertain how review Submissions: Accord, PGA Panel found no indication that a review Chapter 7 - Avenues mechanism would operate Forums and meetings mechanism is required for review Efficiency of scheduling (Recommendation 8). process should not be reduced Publication of interim decision provides an avenue to review the proposed decision As no avenues for review, Submissions: ADA, Accord, ASMI Panel noted that secretariat and delegates Chapter 4 - System greater transparency Forums and meetings are considering the level of detail to be of access needed included in a notice of decision. controls/Clarity and Panel recommended that level of detail, transparency of clarity and transparency contained in process to amend public notices is improved the Poisons (Recommendation 1). Standard
Other matters
Issue raised Source Response Report reference
Scope of review should Submissions: ASMI For matters that were broader than the Chapter 8 - Other encompass all aspects of Forums and meetings current operation of Part 6-3 the panel matters scheduling made a number of observations throughout the report and in Chapter 8. Issue raised Source Response Report reference
Better integration with Submissions: CMIC, CHC, Accord, Panel noted effect on industry. Chapter 6 - Effects other parts of regulation, ASMI, APVMA, CHF The panel found that scheduling and of 2009 policy and processes Forums and meetings other relevant policies and processes amendments on should be aligned to the extent possible industry/Process and encouraged further work to achieve issues Chapter 8 - this. Other matters /Role, profile and integration of scheduling with other parts of regulation policy and processes
Lack of profile of Forums and meetings Panel noted that TGA communication Chapter 8 - Other chemicals scheduling and education reforms provide matters/Role, opportunities for increased profile and communication. integration of scheduling with other parts of regulation, policy and processes Issue raised Source Response Report reference
Exclusivity provisions Submissions: ASMI The panel did not review this matter. for rescheduling Exclusivity considerations are relevant applications and regulated for the purpose of access to market through registration/approvals. Pharmacovigilance of Submissions: PSA The panel did not review this matter. rescheduled substances Forums and meetings Mandatory adverse experience reporting mechanisms are already in place for therapeutic products, agricultural and veterinary chemical products and consumer goods. Poor alignment in access Forums and meetings Panel noted complexities arising from Chapter 8 - Other controls inconsistencies in state and territory matters/Importanc approaches to impose regulatory e of consistency in controls on substances. state and territory medicine and chemicals access controls
Certainty regarding Submissions: PGA, ASMI The panel did not review this matter. future work and ongoing The operation of Schedule 2 and 3 is use of Schedule 2 and 3 separate AHMC project and Galbally review recommendation. Issue raised Source Response Report reference
Enhance consumer Submissions: CHF Panel noted that TGA communication Chapter 8 - Other understanding of the Forums and meetings and education reforms provide matters/Role, basis and implications of opportunities for increased profile and scheduling decisions communication. integration of scheduling with other parts of regulation, policy and processes Approaches to Panel contact by overseas researcher The panel considered this to be outside reclassification of its terms of reference and did not medicines in different review this matter. countries Key performance indicators
Issue raised Source Response Report reference
Various suggestions for Submissions: Accord, APVMA Panel encouraged the collection of data Chapter 8 - Other key performance State and territory survey to facilitate any future review of the matters indicators provided Forums and meetings scheduling arrangements.
Acronyms ACCS – Advisory Committee on Chemicals Scheduling ACMS – Advisory Committee on Medicines Scheduling ADA – Australian Dental Association AHMC – Australian Health Ministers’ Conference APVMA – Australian Pesticides and Veterinary Medicines Authority ASMI – Australian Self Medication Industry OBPR – Office of Best Practice Regulation PGA – Pharmacy Guild of Australia PSA – Pharmaceutical Society of Australia RIS – Regulation Impact Statement SPF – Scheduling Policy Framework for Medicines and Chemicals TGA – Therapeutic Goods Administration