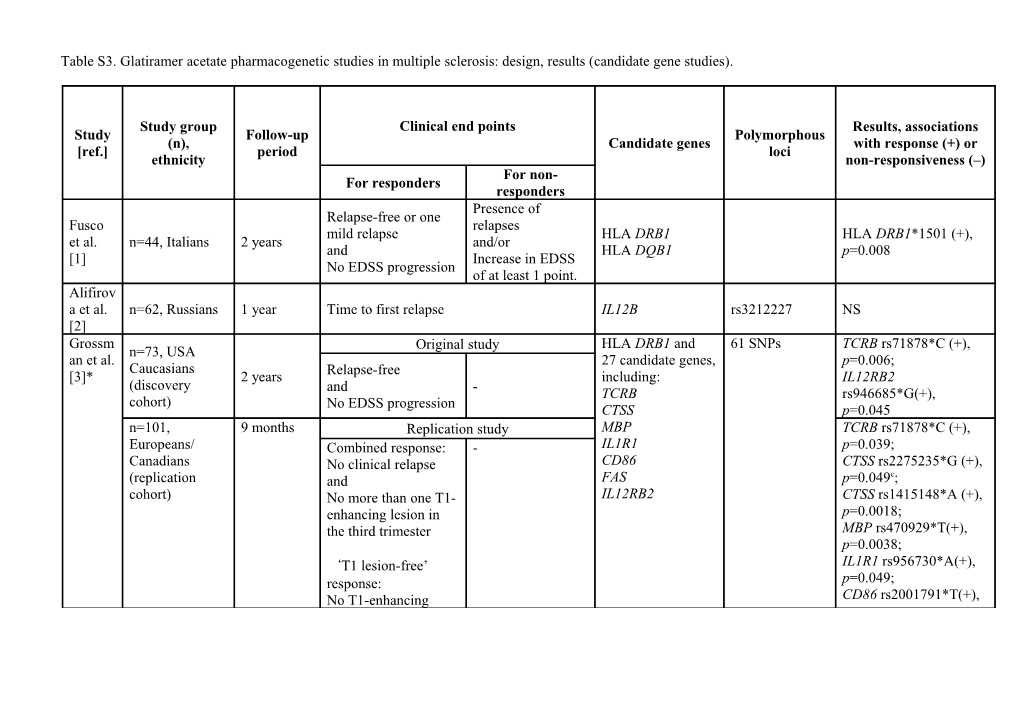
Table S3. Glatiramer acetate pharmacogenetic studies in multiple sclerosis: design, results (candidate gene studies).
Study group Clinical end points Results, associations Study Follow-up Polymorphous (n), Candidate genes with response (+) or [ref.] period loci ethnicity non-responsiveness (–) For non- For responders responders Presence of Relapse-free or one Fusco relapses mild relapse HLA DRB1 HLA DRB1*1501 (+), et al. n=44, Italians 2 years and/or and HLA DQB1 p=0.008 [1] Increase in EDSS No EDSS progression of at least 1 point. Alifirov a et al. n=62, Russians 1 year Time to first relapse IL12В rs3212227 NS [2] Grossm Original study HLA DRB1 and 61 SNPs TCRB rs71878*С (+), n=73, USA an et al. 27 candidate genes, p=0.006; Caucasians [3]* 2 years Relapse-free including: IL12RB2 (discovery and - TCRB rs946685*G(+), cohort) No EDSS progression CTSS p=0.045 n=101, 9 months Replication study MBP TCRB rs71878*C (+), Europeans/ Combined response: - IL1R1 p=0.039; Canadians No clinical relapse CD86 CTSS rs2275235*G (+), c (replication and FAS p=0.049 ; cohort) No more than one T1- IL12RB2 CTSS rs1415148*A (+), enhancing lesion in p=0.0018; the third trimester MBP rs470929*T(+), p=0.0038; ‘T1 lesion-free’ IL1R1 rs956730*A(+), response: p=0.049; No T1-enhancing CD86 rs2001791*T(+), lesions in the third trimester p=0.04; CD86 rs1129055 C (–), p=0.04; Time to first clinical event (clinical relapse, Gross Approximat n=332, changes in MRI lesion burden, increase of rs3135388*A/Aa (+), et al. ely 600 DRB1 rs3135388 Americans EDSS by 1 point, sustained over a 6-month p=0.015/0.048b [4] weeks period) DRB1 HLA class II CTLA4 rs231775 CCR5 rs333 HLA-DRB1*04 (+), Tsareva Relapse free status IL7RA rs6897932 p=0.027; et al. n=285, Russians 2 years and - TNF rs1800629 CCR5 rs333*w/w (+), [5] No EDSS progression IFNB1 rs1051922 p=0.046 IFNG rs2430561 TGFB1 rs1800469 IFNAR1 rs1012335 DRB1 HLA class II CTLA4 rs231775 One or more CCR5 rs333 relapses Tsareva Relapse free status IL7RA rs6897932 and/or et al. n=214, Russians 2 years and TNF rs1800629 NS Increase of at least [6] No EDSS progression IFNB1 rs1051922 1 point in EDSS IFNG rs2430561 over 1 year period TGFB1 rs1800469 IFNAR1 rs1012335 Dhib- n=64, 2 years Relapse-free status One or more HLA-DRB1 DR15 (+), p=0.02; Jalbut Americans and relapses HLA-DQB1 DQ6 (+), p=0.014; et al., No EDSS progression or DR17 (–), p=0.012; [7] Increase of at least DQ2 (–), p=0.002; 1 point in EDSS Haplotypes: DR15-DQ6 (+), p=0.044c DR17-DQ2 (–), p=0.046c 1) One or more Lopez- relapses Relapse-free status Gomez or n=64, Spanish 2 years and TRAILR1 rs20576 NS et al., 2) Increase of at No EDSS progression [8] least 1 point in EDSS NS - not significant; OR – odds ratio; HR – hazard ratio; CI – confidence interval; a reflecting HLA-DRB1*1501/1501 genotype; b p- and HR[95%CI] values (adjusted for baseline confounders) are separated by slash for comparisons of A/G vs A/A and G/G vs A/A; c p-value after multiple comparisons correction. Referemces:
[1] Fusco C, Andreone V, Coppola G, Luongo V, Guerini F, Pace E et al. HLA-DRB1*1501 and
response to copolymer-1 therapy in relapsing-remitting multiple sclerosis. Neurology,
2001;57:1976-9.
[2] Alifirova VM, Orlova I, Babenko SA, Rudko AA, Puzyrev VP. [The 1188 A/C ILI2B gene
polymorphism in patients with multiple sclerosis and in healthy subjects of Tomsk region].
Zh Nevrol Psikhiatr Im S S Korsakova, 2006;Spec No 3:130-5.
[3] Grossman I, Avidan N, Singer C, Goldstaub D, Hayardeny L, Eyal E et al. Pharmacogenetics
of glatiramer acetate therapy for multiple sclerosis reveals drug-response markers.
Pharmacogenet Genomics, 2007;17:657-66.
[4] Gross R, Healy BC, Cepok S, Chitnis T, Khoury SJ, Hemmer B et al. Population structure
and HLA DRB1 1501 in the response of subjects with multiple sclerosis to first-line
treatments. Journal of neuroimmunology, 2011;233:168-74.
[5] Tsareva E, Kulakova OG, Makarycheva O, Boiko AN, Shchur SG, Lashch N et al.
[Pharmacogenomics of multiple sclerosis: association of immune response genes
polymorphism with copaxone treatment efficacy]. Molekuliarnaia biologiia, 2011;45:963-72.
[6] Tsareva EY, Kulakova OG, Boyko AN, Shchur SG, Lvovs D, Favorov AV et al. Allelic
combinations of immune-response genes associated with glatiramer acetate treatment
response in Russian multiple sclerosis patients. Pharmacogenomics, 2012;13:43-53.
[7] Dhib-Jalbut S, Valenzuela R, Ito K, Kaufman M, Picone M, Buyske S. HLA DR and DQ
alleles and haplotypes associated with clinical response to glatiramer acetate in multiple
sclerosis. Multiple Sclerosis and Related Disorders, 2013;2:340-8.
[8] Lopez-Gomez C, Pino-Angeles A, Orpez-Zafra T, Pinto-Medel MJ, Oliver-Martos B, Ortega-
Pinazo J et al. Candidate Gene Study of TRAIL and TRAIL Receptors: Association with
Response to Interferon Beta Therapy in Multiple Sclerosis Patients. PloS one, 2013;8:e62540.