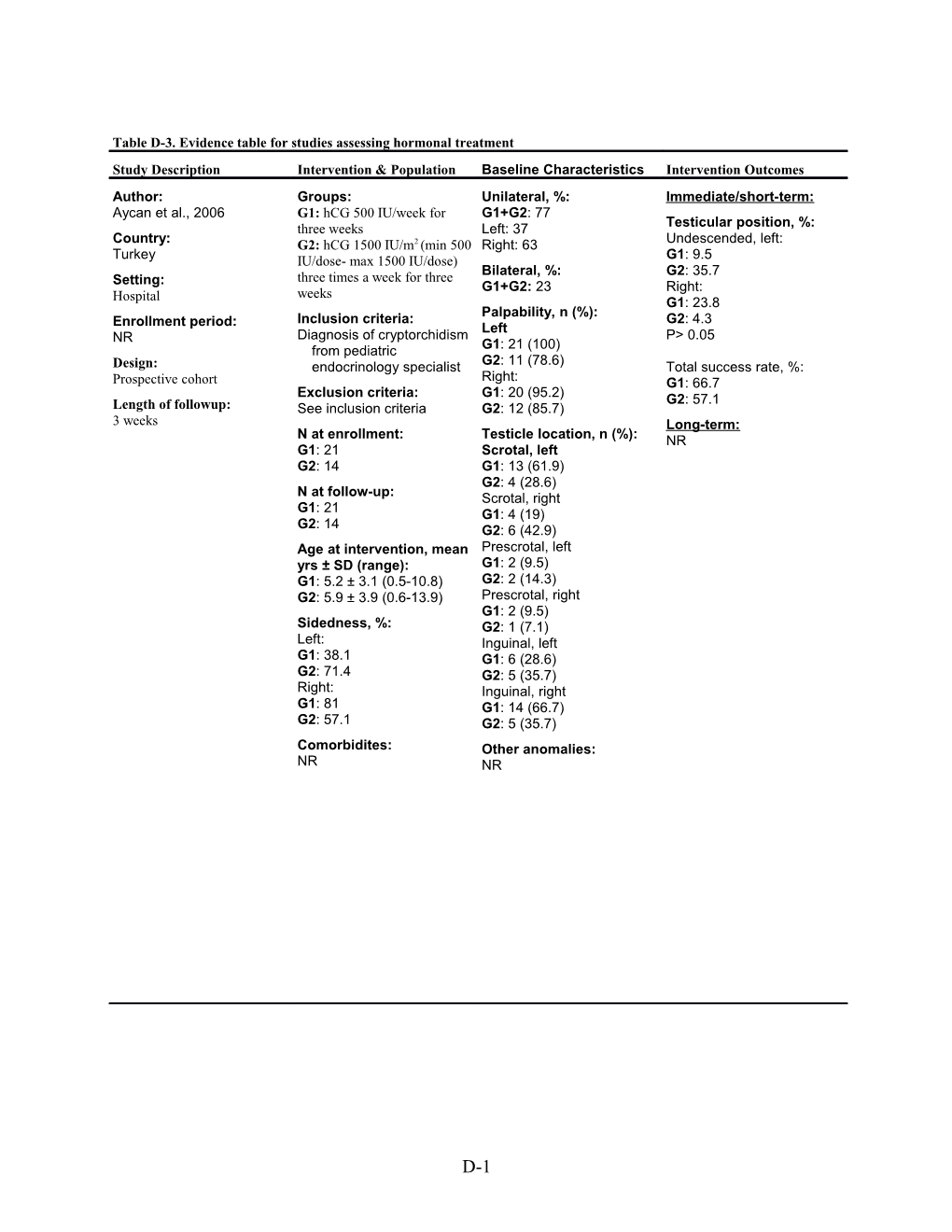
Table D-3. Evidence table for studies assessing hormonal treatment Study Description Intervention & Population Baseline Characteristics Intervention Outcomes Author: Groups: Unilateral, %: Immediate/short-term: Aycan et al., 2006 G1: hCG 500 IU/week for G1+G2: 77 three weeks Left: 37 Testicular position, %: Country: G2: hCG 1500 IU/m2 (min 500 Right: 63 Undescended, left: Turkey IU/dose- max 1500 IU/dose) G1: 9.5 Bilateral, %: G2: 35.7 Setting: three times a week for three weeks G1+G2: 23 Right: Hospital G1: 23.8 Inclusion criteria: Palpability, n (%): G2: 4.3 Enrollment period: Left NR Diagnosis of cryptorchidism P> 0.05 from pediatric G1: 21 (100) Design: endocrinology specialist G2: 11 (78.6) Total success rate, %: Prospective cohort Right: G1: 66.7 Exclusion criteria: G1: 20 (95.2) G2: 57.1 Length of followup: See inclusion criteria G2: 12 (85.7) 3 weeks Long-term: N at enrollment: Testicle location, n (%): NR G1: 21 Scrotal, left G2: 14 G1: 13 (61.9) G2: 4 (28.6) N at follow-up: Scrotal, right G1: 21 G1: 4 (19) G2: 14 G2: 6 (42.9) Age at intervention, mean Prescrotal, left yrs ± SD (range): G1: 2 (9.5) G1: 5.2 ± 3.1 (0.5-10.8) G2: 2 (14.3) G2: 5.9 ± 3.9 (0.6-13.9) Prescrotal, right G1: 2 (9.5) Sidedness, %: G2: 1 (7.1) Left: Inguinal, left G1: 38.1 G1: 6 (28.6) G2: 71.4 G2: 5 (35.7) Right: Inguinal, right G1: 81 G1: 14 (66.7) G2: 57.1 G2: 5 (35.7) Comorbidites: Other anomalies: NR NR
D-1 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Study Description Intervention & Population Baseline Characteristics Intervention Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Bertelloni et al., 2001 G1: hCG 500 IU/week if ≤ 2 G1: 37 (100) years or 1,000 IU/week if > 2 G2: 39 (100) Testicular position: Country: years for 6 weeks G3: 39 (100) Temporary descent Italy G2: hCG 500 IU/week (≤ G4: 40 (100) G1: 8 (21.6) 2years) + hMG 75 IU/week or G2: 7 (17.9) Setting: Bilateral, n: G3: 6 (15.4) Hospital hCG 1000 IU/week (> 2 years) + hMG 75 IU/week for 6 0 G4: 9 (22.5) Enrollment period: weeks Palpability, n (%): 1989-1998 G3: GnRH 1,200 µg/daily for Permanent descent (6 G1: 37 (100) months) n (%) Design: 28 days G2: 39 (100) G4: GnRH 1,200 µg/daily for G1: 7 (18.9) RCT G3: 39 (100) G2: 5 (12.8) 28 days + hCG 1,500 IU/week G4: 40 (100) Length of followup: for 3 weeks G3: 5 (12.8) 6 months post discontinuation Testicle location: G4: 6 (15.0) of therapy Inclusion criteria: Inguinal Unilateral inguinal palpable Pain: G1: 37 (100) Local pain in injection site in testis G2: 39 (100) No clinical evidence of majority of hCG treated G3: 39 (100) boys hernia or other endocrine G4: 40 (100) or syndromic conditions Adverse effects: impairing descent Other anomalies: G1: NR NR Exclusion criteria: G2: NR Retractile testes G3: 0 G4: 0 N at enrollment (N testes): G1: 37 (37) Andronization, n (%): G2: 39 (39) G1+G2+G4: 86 (74.1) G3: 39 (39) G3: 2 (5.1) G4: 40 (40) Long-term: N at follow-up (N testes): NR G1: 37 (37) G2: 39 (39) G3: 39 (39) G4: 40 (40) Age at intervention, range: 10-48 months Sidedness, n (%): Left: 69 (44.5) Right: 86 (55.5) Comorbidites: NR
D-2 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Study Description Intervention & Population Baseline Characteristics Intervention Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Bica and Hadziselimovic G1: Buserelinvia nasal spray G1: 19/22 (86) 1992, 1993 20 µg per day every 8 hours for G2: 19/19 (100) Testicular position: 28 days + 1,500 IU HCG G3: 15/18 (85) Descent, %: Country: intramuscularly once a week G1: 28 Brazil for 3 weeks Bilateral, n (%): G2: 0 G2: Placebo (physiological G1: 3/22 (14) Setting: G2: 0/19 (0) Long-term: Hospital saline solution) nasal spray for 28 days + 1,500 IU HCG G3: 3/18 (17) NR Enrollment period: intramuscularly once a week Palpability: March 1989 to May 1990 for 3 weeks NR G3:Orchiopexy Design: Testicle location, n (%): RCT Inclusion criteria: Abdominal Length of followup: True cryptorchidism G1: 2/25 (8) 3 months Exclusion criteria: G2: 3/19 (16) Retractile testes G3: 3/21 (14) Ectopic testes Inguinal Concomitant hernia G1: 12/25 (45) Unsuccessful previous G2: 7/19 (37) orchiopexy G3: 10/21 (48) Unsuccessful hormone Prescrotal treatment G1: 11/25 (44) Aarskog syndrome G2: 9/19 (47) G3: 8/21 (38) N at enrollment (N testes): G1: 23 (26) Other anomalies: G2: 20 (20) NR G3: 20 (23) N at follow-up: G1: 22 G2: 19 G3: 18 Age at intervention, (n) mean ± SD: G1: (22) 3.7 ± 2.0 G2: (19) 4.3 ± 2.0 G3: (18) 4.8 ± 1.9 Sidedness: NR Comorbidites,: NR
D-3 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Bilateral, rate of descent %* Author: Groups: Unilateral, n (N testes): (retractile testes excluded, Christiansen et al., 1988 G1: hCG 100 IU/kg im G1: 31 (31) n=21): G2: 22 (22) G1: 23 Country: (maximum 1500 IU) G3: 35 (35) Denmark twice weekly for 3 G2: 9 Bilateral, n (N testes): G3: 0 weeks G1: 57 (114) P=0.001 (Fisher’s exact) Setting: G2: GnRH, 200 µg in G2: 52 (104) 6 participating centers and G3: 46 (92) Unilateral, rate of descent private physicians’ offices each nostril three times %*: (retractile testes a day for 28 days Palpability: excluded, n=2): See below G1: 15 Enrollment period: G3: placebo, 200 µg in Testicle location, (%): G2: 0 NR each nostril three times G3: 0 a day for 28 days Not palpable Design: Bi L: 10 P=0.017 (Fisher’s exact) RCT (modified double Inclusion criteria: Bi R: 8 Adverse effects, %: Unilateral or bilateral Uni L: 5 Pain in genital region : blind) cryptorchidism Uni R: 9 G1: 0 Inguinal Length of followup: No previous hormonal G2: 7 treatment Bi L: 64 G3: 1 4-8 weeks No operation in inguino- Bi R: 73 Erections scrotal region Uni L: 34 G1: 14 Retractile testes(testes that Uni R: 41 G2: 1 were spontaneously in Suprascrotal G3: 0 position 0-3 but could be Bi L: 12 Growth of penis manipulated into position Bi R: 12 G1: 7 4) Uni L: 2 G2: 0 Uni R: 7 G3: 0 Exclusion criteria: High Scrotal Pain at site of injection Inguinal hernia Bi L: 14 G1: 4 Ectopic testes Bi R: 7 Nose Bleeding Endocrine or chromosomal Uni L: 1 G2: 1 disorders Uni R: 1 G3: 1 Testes in position 4 at Normal Psychological changes beginning of exam but Uni L: 58 G1: 7 retracted to suprascrotal Uni R: 42 G2: 12 location by strong G3: 10 cremaster muscle Other anomalies: Long-term: N at enrollment : 317 boys NR NR N at follow-up (N testes): Total 243 boys (398) G1: 88 (145) G2: 74 (126) G3: 81 (127) Age at intervention, years, n (range): Bilateral : 155 (1.8 – 13.0 ) Unilateral: 88 (1.5-13.1 ) Sidedness: See testicle location Comorbidites: NR *Percentage is rate of descent without retractile testes
D-4 D-5 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: DeMuinck Keizer -Schrama G1: Synthetic LHRH G1+ G2: 203 (80.6) et al., 1986, 1987 and Testicular position: Hazebroek et al, 1987 intranasal 1.2 mg/day, Bilateral, n (%): Descent after 8 weeks, n one 200µg puff in G1+G2: 49 (19.4) (%): Country: G1: 14 testicles (9) Netherlands each nostril three times Palpability, n (%): G2: 10 testicles (8) a day before meals for NR After open study: Setting: G1: 13 Hospital 4 weeks Testicle location: NR G2: 21 Enrollment period: G2: Placebo Complete descent after 8 October 1982 to April 1985 Other anomalies: weeks, n (%): Results evaluated at 8 NR G1A: 1/30 (3) Design: G2A: 0/25 (0) weeks. All non- RCT followed by open G1B: 1/55 (2) responders were G2B: 6/52 (11) cohort offered additional G1C: 12/66 (18) G2C: 4/53 (7) Length of followup: treatment with LHRH. Complete descent after two 6 months to 2 years Failure of hormonal courses of LHRH in G1 and treatment followed by G2 together n (%) Ga: 4 (7) surgical intervention: Gb: 12 (12) 170 boys (196 testes)* Gc: 32 (28) Ga: 36 Total: 48 (18) Late descent, n (%) Gb: 72 Ga: 3 (5) Gc: 62 Gb: 1 (1) Gc: 10 (9) Inclusion criteria: Total: 14 (5) Cryptorchidism (one or both testes not located in or Need for further surgical could not be manipulated intervention: fully into the scrotum) G1+G2: 170 (196 testes) Bilateral 26 Exclusion criteria: Unilateral 144 (69 right, 75 Previous hormonal or left) surgical treatment for cryptorchidism Long-term: Retractile testes NR Truly ectopic testicular positions (perineal, penile, etc) Concomitant inguinal hernia Chromosomal or dysmorphic syndromes N at enrollment (N testes): G1 + G2: 252 (301) Ga + Gb + Gc: 170 (196) N at follow-up: G1: 151 G2: 130 Age at intervention, mean yrs ± SD: 5.6 ± 3.3
D-6 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes
Sidedness, n (%): Left: G1+G2: 99 (48.8) Right: G1+G2: 104 (51.2) Comorbidites: NR
D-7 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Study Description Intervention & Population Baseline Characteristics Intervention Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Esposito et al., 2003 G1: hCG 500 IU i.m. twice a 230 (71.0) week for 6 weeks Left: 111 (48.3) Testicular position: Country: G2: hMG 150 IU i.m. twice a Right: 119 (51.7) Descent, n %: Italy week for 4 weeks G1: 39/113 (34.5) G3: LH-RH nasal spray 1.2 Bilateral, n (%): G2: 0/35 (0) Setting: 94 (29.0) G3: 25/85 (29.4) Hospital mg/day for 4 weeks G4: hMG 150 IU i.m. twice a G4: 7/27 (25.9) Palpability, n (%): G5: 19/64 (29.6) Enrollment period: week for 4 weeks followed by G1-G5: (100) January 1997 to June1999 hCG 500 IU i.m. twice a week Testicle location, %: Total: 90 (27.7) Design: for 6 weeks G5: LH-RH nasal spray 1.2 Inguinal: 100 Bilateral: 36 (38.2) RCT Unilateral: 54 (23.4) mg/day for 4 weeks followed Other anomalies: Length of followup: by hCG 500 IU i.m. twice a NR 4-10 weeks week for 6 weeks P=0.007 Inclusion criteria: Need for further surgical Testes palpable in the intervention: inguinal canal 14 (4.3) Exclusion criteria: Adverse effects: Retractile (non-scrotal G1, G4, G5: frequent testes that could be erections, aggressive manipulated into the behavior, development of bottom of the scrotum but pubic hair, pain at injection immediately retracted to site or inguinal area (n not initial prescrotal upon reported) release) Long-term: Non-palpable testes NR N at enrollment: G1: 113 G2: 35 G3: 85 G4: 27 G5: 64 N at follow-up: G1: 113 G2: 35 G3: 85 G4: 27 G5: 64 Age at intervention, media yrs, (range): 3.5 (1.2-6) Sidedness: NR Comorbidites: NR
D-8 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Forest et al, 1988 G1: hCG (1,500 G1: 263 (60) G1a: 204 (58) Testicular position: Country: IU/injection) 7 G1b: 59 (67) Successful descent France intramuscular G2: 57 (60) unilateral G1: 107 (40.7) Setting: injections every other Bilateral, n (%): G1a: 77 (37.8) Clinic day G1: 177 (40) G1b: 30 (50.8) Enrollment period: G1a: Retrospective G1a: 148 (42) G2: 29 (50.9) 1983 to 1988 G1b: 29 (33) study (n=352) G2: 38 (40) Success in bilateral Design: G1b: Prospective study G1: Palpability, n (%): NR RCT with prospective (n=88) One side 42 G1: 123 Both sides 88 and retrospective G2: 4 intramuscular G2: 123 Total 130 (36.7) aspects injections of hCG in Testicle location: G1a: dose related to body Abdominal One side 34 Length of followup: Both sides 68 weight (100 IU/kg) to a G1a: 320 NR G1b: 50 Total 102 maximum dose of G2: 56 G1b: 3,000 IU) at 4 day Inguinal One side 8 G1a: 180 Both sides 20 (G2a) or 5 day (G2b) G1b: 67 Total 28 intervals G2: 77 G2: One side 52 Inclusion criteria: Other anomalies: Both sides 116 Undescended testes NR Total 168 (39.1) Exclusion criteria: Long-term: Retractile testes Inguinal hernia Endocrine function: Overt endocrine Testosterone levels, mean ± disturbances SD (median): G1a: 5.86 ± 2.89 (5.25) N at enrollment: ng/ml G1: 440 20.3 ± 10 (182) nmol/l G1a: 352 G1b: 5.16 ± 2.73 (4.43) G1b: 88 ng/ml G2: 95 17.9 ± 9.5 (15.4) nmol/l N at follow-up: G2: 4.08 ± 2.07 (3.84) ng/ml G1: 440 14.2 ± 7.2 (13.3) nmol/l G2: 95 Age at intervention: G1b+G2: range 7 months- 12 years Sidedness, n (%): Left: G1: 118/263 G2: 18/57 Right: G1: 145/263 G2: 39/60 Comorbidites: NR
D-9 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Hadziselimovic, 2008 G1: Schoemakers type of 30 (100) NR Country: orchiopexy between ages 1-6 Bilateral, n: Long-term: Switzerland years subsequently treated 0 within 3 months after surgery Testicular size and Setting: with LH-RH, 10 µg applied as Palpability: appearance: Hospital, clinic intranasal spray in the evening NR G1: on alternate dats for 6 months Cryptorchid testis 1.2(95% Enrollment period: Testicle location: CI 1-3.2) G2: Age matched controls who NR NR had undergone successful P=0.65 Schoemaker type orchiopexy Other anomalies: Descended testis 1.4 (95% Design: with testicular biopsy results NR CI 1-2.5) Retrospective case and no additional LH-RH P=0.52 series treatment Testicular volume Penis length 5.0 cm (CI 4.5- G1: 6) Length of followup: Inclusion criteria: Crytorchid testis 1.4 (95% P<0.001 Unilateral cryptorchid boys CI 0.8-2.1) 15-19 years following located outside of Descended testis 1.2 (95% Germ cells, average # per initial treatment scrotum since birth CI 1-3.2) tubular cross section: G1: No additional surgeries Penis length 4.5cm (CI 4-5) G1: 0 (95% CI 0- 0.05) or severe illnesses G2: 0.02 (95% CI 0-0.05) requiring hospitalization Ad spermatogonia at P=0.22 during the 15-19 years surgery following treatment; no Infertility/subfertility: G1: 0 Sperm count/ejaculate chronic medication use or G2: 0 drug abuse; (mio): G2: no Ad spermatogonia G1: 90 (95% CI 53-164) S/T at surgery G2: 1.0 (95%CI 0-13) and total # germ cells < G1: 0 (95%CI 0-0.05) 0.2 per tubule P≤ 0.0001 G2: 0.02 (95% CI 0-2) Ejaculate volume (mL) Exclusion criteria: p=0.22 G1: 4.1 (95% CI 1.2-7) See inclusion criteria G2: 4.6 (95% CI 2.9- 8.2) Unsuccessful HCG N at enrollment (N testes): P=0.074 treatment prior to surgery Normal morphology G1: 15 (15) G2: 13/15 G2: 15 (15) G1: 11% (95%CI 0-21) G2: 0 N at follow-up (N testes): G1: 15 (15) G2: 15 (15) Age at intervention, mean yrs (range): G1: 3 (1-6) G2: 4 (NR) Age at spermiogram, mean yrs: G1: 19 G2: 21 P< 0.02 Sidedness: NR Comorbidites:NR
D-10 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral: Immediate/short-term: NR Hadziselimovic et al., 1997 G1: Previously G1: 6 G2: 13 NR Country: underwent orchiopexy Long-term: Switzerland after failure to respond Bilateral, n: G1: 4 Setting: Infertility/subfertility: to hCG treatment, then G2: 10 Spermiogram results: NR received long-term Palpability, n (%): Increase in number of Enrollment period: treatment of buserelin NR spermatozoa, increased NR number of normal forms of nasal spray 10µg every Testicle location: spermatozoa per ejaculate, Design: other day for 6 months NR improved sperm motility in after successful G1 Retrospective cohort Other anomalies: Number of sperm: orchiopexy NR Length of followup: G1: 29.4 G2: No hormone G2: 6.5 NR treatment after P<0.005
orchiopexy Percent of normal sperm: Inclusion criteria: G1: 31.8 Previously underwent G2: 15.2 orchiopexy P < 0.03
Exclusion criteria: Percent of motile sperm None had secondary G1: 41.3 testicular ascent, previous G2: 11.2 inguinal surgery before P<0.001 orchiopexy, congenital malformation, or long- standing illness N at enrollment (N testes): G1: 10 (14) G2: 23 (33) N at follow-up (N testes): G1: 10 (14) G2: 23 (33) Age at study, mean yrs ± SD: G1: 22.1 ± 2.07 (underwent orchiopexy at 9.4 ± 2.8) G2: 20.9 ± 2.5 Sidedness: NR Comorbidites: NR
D-11 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral: Immediate/short-term: Hagberg and Westphal, G1: Two nasal applications NR 1982 of 100 µg LH-RH with an Testicular position: interval of 30-60 minutes 3 Bilateral: Therapeutic effect: Country: times a day for 28 days NR G1: 18/29 testes (3 non- Sweden (total daily dose 600 µg) palpable moved to inguinal; Palpability: 7 inguinal moved to scrotal Setting: G2: Placebo nasal NR neck; 2 inquinal and 6 Hospital spray for 28 days Testicle location: scrotal neck- complete Enrollment period: G2a: Subsequently NR descent) NR G2: 1/32 testes placebo treated with LH-RH Other anomalies: G2a: 19 testes moved (1 Design: after 28 days NR nonpalpable moved to RCT inguinal; 1 nonpalpable Inclusion criteria: moved to scrotal neck; 9 Length of followup: Undescended testes inguinal moved to scrotal 4 weeks to 12 months Exclusion criteria: neck; 5 inguinal and 3 Testes that could be scrotal neck – complete manipulated to bottom of descent) scrotum even if spontaneous location was G1+G2a combined (n=46, in the scrotal neck 60 testes): 4 palpable moved to inguinal; 1 non N at enrollment (N testes): palpable moved to scrotal G1: 25 neck; 16 inguinal moved to G2: 25 scrotal neck; 7 inguinal and N at follow-up (N testes): 9 scrotal neck – complete G1: 23 (29 testes) descent. G2: 24 (32 testes) 5 non palpable, 10 inguinal and 8 scrotal neck Age at intervention, mean unchanged. yrs (range): 5 (1.5 – 10.5) Follow-up study 6-12 months after LH-RH Sidedness: treatment in 23 cases with NR initial descent from inguinal to scrotal position Comorbidites: 18 testes remained NR completely descended 2 inguinal relapsed to scrotal neck and 3 relapsed to inguinal Adverse effects, n: More active G1: 3 G2:1 G2a:6 More aggressive G1: 1 G2: 1 G2a: 6 Furunculosis G1: 0 G2: 0 G2a: 2
D-12 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes
Impetigo G1: 0 G2: 0 G2a: 1 Local symptoms of nasal application G1: 1 G2: 1 G2a: 0 Long-term: NR
D-13 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%)*: Immediate/short-term: Hesse and Fischer, 1988 173 (52.1) G1: hCG 300-1000 IU, Testicular position: Country: 2 IM injections/week Bilateral, n (%)*: Scrotal descent, %: Germany G2: hCG 1000-500 IU, 163 (49.1) G1: 39.4 G2: 29.9 Setting: IM injection every 7- Palpability: Hospital 10 days NR Long-term: NR Enrollment period: Testicle location, n: NR Inclusion criteria: NR Abdominal: See exclusion criteria G1:54 Design: Exclusion criteria: G2:37 RCT Treatment continued outside Inguinal: catchment area G1:134 Length of followup: G2:114 N at enrollment (N testes): 8 wks to 6 months Retractile: 395 (NR) G1: 15 N at follow-up (N testes): G2: 41 332 (435) Scrotal G1: 163 (NR) G1:39 G2: 169 (NR) G2l: 46 Age at intervention range Other anomalies: yrs: NR 1-13 Sidedness: NR Comorbidites: NR
D-14 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Karpe et al., 1983 G1: LHRH (HOE 471) G1: 25 (100) G2: 25 (100) Testicular position: Country: intranasal spray 100µg Descent after treatment, n Sweden in each nostril 6 times Bilateral, n: (%): 0 G1: 5 (20.0) Setting: a day, minimum time (3 complete, 2 borderline) Hospital lapse of 1 hour Palpability: G2: 3 (12.0) NR Enrollment period: between doses for 28 6 months later NR Testicle location: G1: 2 (8.0) days NR G2: 1 (4.0) Design: G2: Placebo RCT Other anomalies: Mean basal serum Inclusion criteria: NR testosterone: Unilateral undescended but after treatment Length of followup: palpable testes Mean basal serum testosterone: G1: 58.9 ± 31.7 pmol/L 6 months No previous treatment for G2: 34.9 ± 20.9 pmol/L undecended testis G1: 36.9 ± 17.3 G2: 31.7 ± 11.5 Exclusion criteria: Post treatment FSH peak Retractile testes decreased in significant # of Any sign of hernia patients (p<0.001) Previous inguinal surgery Need for further surgical N at enrollment (N testes): intervention, n (%): G1: 25 (25) 39 (78.0) G2: 25 (25) Adverse effects, n (%): N at follow-up (N testes): Sparse moustache:1 G1: 25 (25) Hydrocele of tunica G2: 25 (25) vaginalis: G1: 3 (12.0) Age at intervention, mean G2: 0 yrs ± SD (range): 6.3 ± 1.4 (3-8) Bilateral increase of < 1ml Sidedness: testicular volume occurred NR in significant number of G1 followed by decrease during Comorbidites: months after treatment (p< NR 0.001) Long-term: NR
D-15 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n: Immediate/short-term: Olsen et al, 1992 G1: LHRH nasal spray G1: 27 G2: 32 Testicular position: Country: (0.4 mg three times Full response for non Denmark daily) Bilateral, n: descended testes, n G1: 35 participants (%) Setting: G2: Placebo nasal G2: 29 G1: 6/62 (9.7) Hospitals spray three times daily G2: 1/61 (1.6) Palpability: Enrollment period: Therapeutic gain of G1: Inclusion criteria: NR NR 8.1% (95% CI 0.1-16.6%) At least one non descended Testicle location: p=0.12 testis at clinical Design: Non palpable, n testes: examination G1: 7 Unilateral non-descended, n RCT No former treatment for non- G2: 9 participants (%): descended testis Inguinal G1: 1/27 (3.7) Length of followup: No clinical signs of G1: 76 G2: 1/32 (3.1) 2 weeks (not including endocrine disease G2: 73 Therapeutic gain of G1 No clinical signs of 4 week intervention) High scrotal 0.6% (95% CI 0.0-16.1) beginning puberty G1: 14 p=1.0 No former surgery in the G2: 8 inguinal area Bilateral non descended- No clinical sign of inguinal Other anomalies: both descended, n hernia or ectopia NR participants (%): Age ≥ 2 years G1: 5/35 (14.2) Retractile (testes located in G2: 0/29 (0) high scrotal position but Therapeutic gain of G1 could be manipulated into 14.3% (95% CI 2.7-25.1) bottom of scrotum ) p=0.09 Exclusion criteria: Testes remaining non- Total full response, n testes palpable after (%) manipulation and caudal G1: 19/97 (19.6) traction in the inguinal G2: 2/90 (2.2) region or bilateral Therapeutic gain of G1: retention on both sides 17.4% (95% CI 8.9-25.8, p=0.0002) N at enrollment: G1: 70 Bilateral non descended G2: 71 (n=128) N at follow-up (N testes): G1: 18/70 (25.7) G1: 62 (97) G2: 1/58 (1.7) G2: 61 (90) Therapeutic gain of G1: 24.0% (95% CI 13.2-34.8, Age at intervention, p=0.0001) median yrs (range): 6 (2-12) Need for further surgical intervention: Sidedness: G1+G2: 74 (95 testes) NR Adverse effects: Comorbidites: Reversible changes in NR behavior G1: 0 G2: 2 Long-term: NR
D-16 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n: Immediate/short-term: Rajfer et al, 1986 G1: Intranasal GnRH G1: 15 G2: 14 Testicular position: Country: spray 100 µg + placebo Descent, n: US injection Bilateral, n: G1: 3 G1: 1 G2: 1 Setting: G2: hCG 3300 IU per G2: 3 Hospital 1.65 ml injection + Endocrine: Palpability: Serum LH levels: Enrollment period: placebo nasal spray NR NR 30 minutes after 1st Inclusion criteria: Testicle location: treatment: Design: Cryptorchidism- NR G1: 9.4 ± 1.3 (n=16) RCT intraabdominal (non 60 minutes after 1st palpable) intracanalicular, Other anomalies: treatment Length of followup: emergent at external NR G1: 8.6 ±1.3 (n=15) inguinal ring or ectopic in LH level, mean ± SD: 3 months (after superficial pouch Adverse effects, n: treatment) 2.7 ± 0.2 (n=16) Increase in penile size: 7 Exclusion criteria: Testosterone level, mean Increase in testicular size: 4 Retractile testes ± SD: Scrotal redness: 2 Endocrine disorder G1: 15.4 ± 2.4 Increase in erections: 2 G2: NR Demonstrated aggressive N at enrollment (N testes): behavior: 2 G1: 16 (17) G2: 17 (20) Long-term: N at follow-up (N testes): NR G1: 16 (17) G2: 17 (20) Age at intervention, range yrs: 1-5 Sidedness: NR Comorbidites: NR
D-17 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes Author: Groups: Unilateral, n (%): Immediate/short-term: Wit et al., 1986 G1: 400 µg LHRH in G1: 17 (65.4) G2: 12 (52.2) Testicular position: Country: nasal spray 3 times a 4 weeks Netherlands day for 4 weeks Bilateral, n (%): To the scrotum G1: 9 (34.6) G1: 1 (3) Setting: G2: Spray with no G2: 11 (47.8) G2: 2 (6) Hospital LHRH To scrotal neck Palpability: G1: 3 (9) Enrollment period: NR NR G2: 0 (0) Code was broken after Testicle location: To inguinal canal Design: 8 weeks. LHRH NR G1: 6 (17) RCT followed by open offered for second G2: 7 (21) Other anomalies: Unchanged label course to both groups. NR G1: 21 (60) Length of followup: After 1 or 2 G2: 23 (68) unsuccessful LHRH LH (IU/l), median (range) Impalpable RCT: 8 weeks 0 G1: 4 (11) Open label: 12 months treatments, surgery or G1: 1.3 (1.0-3.4) G2: 2 (6) hGH therapy proposed. G2: 1.3 (<1.0-1.8) 30 8 weeks Inclusion criteria: G1: 5.3 (1.0-20.0) To the scrotum Unilateral or bilateral G2: 6.3 (1.8-18.2) G1: 3 (9) cryptorchidism (one or 60 G2: 0 (0) both testes are not G1: 4.05 (2.0-16.0) To scrotal neck localized in or cannot be G2: 4.2 (1.6-11.5) G1: 2 (6) moved into the lower part FSH (IU/l) median (range) G2: 2 (6) of the scrotum) 0 To inguinal canal G1: <0.9 (<0.9-2.1) G1: 8 (23) Exclusion criteria: G2:1.0 (<0.9-2.2) G2: 4 (12) Patients with one of the 30 Unchanged indications for primary G1: 4.0 (0.9-5.6) G1: 20 (57) surgical intervention G2: 3.3 (2.0-8.5) G2: 26 (76) Congenital syndromes 60 Impalpable Previously treated for G1: 4.0 (1.0-7.1) G1: 2 (6) cryptorchidism G2: 3.5 (1.4-9.7) G2: 2 (6) N at enrollment (N testes): Testosterone (nmol/l) G1: 26 (35) Mean, median (range) Change in position of G2: 23 (34) G1: 0.20 0.17 (0.08-0.50) undescended testes at 8 G2: 0.17 0.17 (<0.05-0.40) weeks# ,mean cm ± SD N at followup (N testes), 8 SHBG (nmol/l) (number testes): weeks: Mean ±SD Median (range) Supine before caudal G1: 26 (35) G1: 79±20 80 (46-134) traction G2: 23 (34) G2: 80±27 84 (23-123) G1 (unilateral): 0.9±1.4 (17) N at followup, 12 months: G2 (unilateral): 0.3±0.9 (12) 48 G1 (bilateral): 0.5±1.7 (18) G2 (bilateral): 0.4±1.7 (22) Age at intervention Supine during caudal mean yrs (range): traction 5.9 (1.2-11.9) G1 (unilateral): 1.1±1.2 (17) G2 (unilateral): 0.7±1.3 (12) Sidedness: G1 (bilateral): 1.5±1.7 (18) NR G2 (bilateral): 0.3±1.1 (20) Comorbidites: Squatting without traction NR G1 (unilateral): 1.0±1.6 (17) G2 (unilateral): 0.4±0.9 (11) G1 (bilateral): 1.0±1.0 (17) G2 (bilateral): 0±1.9 (19)
D-18 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes
Endocrine function: LH (IU/l), median (range) 0 G1: 4.7(1.0-11.7)* G2: 1.2 (<1.0-1.8) 30 G1: 7.5 (4.2-15.3)* G2: 5.1 (1.8-16.6) 60 G1: 4.8 (2.6-11.1) G2: 3.0 (1.8-11.4) FSH (IU/l) median (range) 0 G1: 1.2 (<0.9-3.4)* G2: 0.9 (<0.9-2.5) 30 G1: 2.4 (<0.9-4.9)* G2: 2.8 (<0.9-6.7) 60 G1: 2.0 (<0.9-4.9)* G2: 3.3 (<0.9-7.7) Testosterone (nmol/l) Mean, median (range) G1: 0.39 0.21 (0.07-2.10) G2: 0.16 0.13 (<0.05-0.65) G2:after LHRH treatment 0.36 0.30 (0.05-1.20) SHBG (nmol/l) Mean ±SD Median (range) G1: 76±24 76 (39-141) G2: 77±26 80 (31-115) G2 after LHRH treatment 75±22 75 (49-109)* *P < 0.01 compared to baseline Adverse effects: Aggressive behavior G1: 23% G2: 0 Long-term: Testicular size and appearance: No changes observed in testicular volume Testicular position: One year: Bilateral n=19 (38 testes) 21 testes did not descend 17 showed some descent but 7 re-ascended 23 testes were operated on Unilateral n=29 (29 testes) 4 testes descended to retractile position
D-19 Table D-3. Evidence table for studies assessing hormonal treatment (continued) Intervention & Intervention Study Description Population Baseline Characteristics Outcomes 23 testes were operated on
D-20