HSC CHEMISTRY PROGRAM
MODULE: OPTION 3 FORENSIC CHEMISTRY
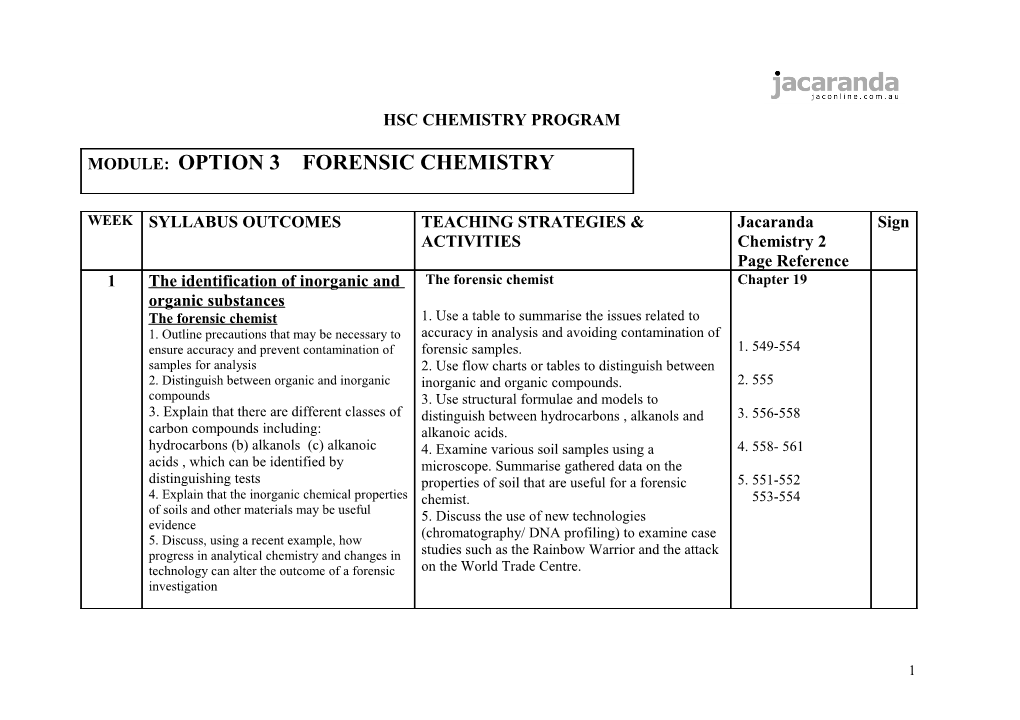
WEEK SYLLABUS OUTCOMES TEACHING STRATEGIES & Jacaranda Sign ACTIVITIES Chemistry 2 Page Reference 1 The identification of inorganic and The forensic chemist Chapter 19 organic substances The forensic chemist 1. Use a table to summarise the issues related to 1. Outline precautions that may be necessary to accuracy in analysis and avoiding contamination of ensure accuracy and prevent contamination of forensic samples. 1. 549-554 samples for analysis 2. Use flow charts or tables to distinguish between 2. Distinguish between organic and inorganic inorganic and organic compounds. 2. 555 compounds 3. Use structural formulae and models to 3. Explain that there are different classes of distinguish between hydrocarbons , alkanols and 3. 556-558 carbon compounds including: alkanoic acids. hydrocarbons (b) alkanols (c) alkanoic 4. Examine various soil samples using a 4. 558- 561 acids , which can be identified by microscope. Summarise gathered data on the distinguishing tests properties of soil that are useful for a forensic 5. 551-552 4. Explain that the inorganic chemical properties chemist. 553-554 of soils and other materials may be useful 5. Discuss the use of new technologies evidence (chromatography/ DNA profiling) to examine case 5. Discuss, using a recent example, how progress in analytical chemistry and changes in studies such as the Rainbow Warrior and the attack technology can alter the outcome of a forensic on the World Trade Centre. investigation
1 6. Solve problems and use available evidence to 6. Solve problems and discuss issues related to 6. 549-550 discuss the importance of accuracy in forensic accuracy in forensic investigations. chemistry 7. Discuss the ethical issues that need to be 7. 550-551 7. Solve problems and use available evidence to addressed in forensic investigations discuss ethical issues that may need to be 8. PRACTICAL ACTIVITY 19.1- 8. 593 addressed during an analytical investigation Distinguishing tests for common inorganic and 8. Identify data, plan and perform first-hand organic compounds. 9. 600-603 investigations to determine a sequence of tests 9. DATA ANALYSIS 19.7 - Comparison of tests to distinguish between organic and inorganic for selected organic and inorganic substances in compounds the school laboratory and the forensic 9. Gather and process information from laboratory. secondary sources to present information summarising a series of distinguishing tests to separate: (a) the groups of hydrocarbons (b)acids, bases and neutral salts ,in the school Revision Questions - Jacaranda Chemistry 2. Set Revision 19.1 laboratory and in the forensic chemist’s 19.1 Pages 561-562 laboratory
2 Analysis of carbohydrates Analysis of carbohydrates Chapter 19 10. Identify that carbohydrates are 10. Use models or structural formulae to verify that 10. 563 composed of carbon, hydrogen and oxygen carbohydrates can be represented by the formula: according to the formula : Cx (H2O)y Cx (H2O)y 11. 563- 569 11. Identify glucose as a monomer and 11. Use diagrams and models to identify describe the condensation reactions which monosaccharides, disaccharides and produce: (a) sucrose as an example of a polysaccharides. Identify glucose as the monomer disaccharide (b) polysaccharides including in starch and cellulose. glycogen, starch and cellulose
2 12. Describe the chemical difference 12. Compare the ring and open chain structure of 12. 569-571 between reducing and non-reducing sugars glucose and sucrose and explain why only glucose 13. Distinguish between plant and animal is a reducing sugar. 13. 566-569 carbohydrates’ composition in terms of the 13. Use diagrams to distinguish between cellulose, presence of: (a)cellulose (b)starch starch and glycogen 14. 594 (c)glycogen 14. PRACTICAL ACTIVITY 19.2(a)- Testing 14. Choose equipment, plan and perform a for reducing and non-reducing sugars and 15. 594-595 first-hand investigation to carry out a series starch of distinguishing tests for the carbohydrates: 15. PRACTICAL ACTIVITY 19.2(b)- Modelling (a) reducing and non-reducing sugars monosaccharides and starch (b)starch 15. Use available evidence and perform first-hand investigations using molecular model kits, computer simulations or other multimedia resources to compare the Revision Questions - Jacaranda Chemistry 2. Set Revision 19.2 structures of organic compounds including: 19.2 Pages 572-574 (a) monosaccharides (b) starch
3 Analysis of proteins Analysis of proteins Chapter 19 16. Distinguish between protein used for 16. Compare , with the aid of diagrams, structural 16. 577-579 structural purposes and the uses of proteins as protein and globular protein. enzymes 17. Use diagrams and models to identify the 17. 574 17. Identify the major functional groups in an functional groups in an amino acid. amino acid 18. Use tabulated information to compare the 18. 575-576 18. Describe the composition and general structures of various amino acids. Identify proteins formula for amino acids and explain that proteins are chains of amino acids as polypeptides. 19. 579-582 19. Describe the nature of the peptide bond and 19. Draw diagrams and construct models of the explain that proteins can be broken at different peptide bond. Solve problems related to the lengths in the chain by choice of enzyme cleavage of the protein chain by selected enzymes.
3 20. Compare the processes of chromatography 20. Use gathered data to compare and contrast 20. 582- 588 and electrophoresis and identify the properties chromatography and electrophoresis. Discuss the of mixtures that allow them to be separated by properties of amino acids and proteins that allow 21. 587-588 either of these processes separation by either procedure. 21. Discuss the role of electrophoresis in 21. Explain how forensic chemists utilise identifying the origins of protein and explain electrophoresis in investigations how this could assist the forensic chemist 22. Perform first-hand investigations using 22. PRACTICAL ACTIVITY 19.3- Modelling 22. 595-596 molecular model kits, computer simulations or the structure of proteins other multimedia resources to present information which describes the composition and generalised structure of proteins
4 Analysis of proteins Analysis of proteins Chapter 19 23. Perform a first-hand investigation and gather first-hand information about a distinguishing 23. Discuss the chemical basis of the Biuret test and 23. 579, 596 test for proteins the ninhydrin test for amino acids and proteins. 24. Perform a first-hand investigation to carry PRACTICAL ACTIVITY 19.4- Distinguishing 24. 583-584 out chromatography to separate a mixture of tests for proteins organic materials such as the pigments in plants 24. Discuss the chromatographic procedure used to 25. Perform a first-hand investigation and gather separate leaf pigments. 25. 597-598 first-hand information to identify the range of 25. PRACTICAL ACTIVITY 19.5- solvents that may be used for chromatography Chromatography: selecting solvents to extract 26. 587- 588 and suggest mixtures that may be separated and pigments from plant leaves. 598-599 identified by the use of these solvents 26. Describe the process of paper and gel 26. Perform a first-hand investigation to carry electrophoresis of proteins and amino acids out the electrophoresis of an appropriate PRACTICAL ACTIVITY 19.6 - mixture and use available evidence to identify Electrophoresis simulations and animations Revision 19.3 the characteristics of the mixture which allow it Revision Questions - Jacaranda Chemistry 2. Set Pages 589-590 to be separated by this process. 19.3
4 5 Instrumental Methods in Forensic DNA and forensic chemistry Chapter 20 Chemistry DNA and forensic chemistry 27. Outline the structure and composition of 27. Use photos, models and drawings to analyse the 27. 605- 608 DNA structure of DNA in terms of its phosphate, sugar 28. Explain why analysis of DNA allows and nitrogen bases. 28. 608 identification of individuals 28. Explain the unique nature of DNA to each 29.Describe the process used to analyse individual in terms of exons and introns. 29. 608- 614 DNA and account for its use in: (a) 29. Describe DNA profiling and use diagrams to identifying relationships between people summarise the steps in creating a DNA profile. 30. 614-617 (b) identifying individuals 30. Summarise the uses of DNA profiling and 30.Analyse information to discuss the range of discuss the ethical issues involved in maintaining uses of DNA analysis in forensic chemistry and DNA databanks. use available evidence in discussing the issues associated with its use in terms of the ethics of maintenance of data banks of DNA Revision Questions - Jacaranda Chemistry 2. Set Revision 20.1 20.1 Pages 617-618
Sensitive analytical techniques Sensitive Analytical Techniques 31. Describe examples of destructive and non- 31. 619- 620 31. Explain what is meant by the destructive testing of material and explain why this may be destructive forensic testing. a problem in forensic investigations 32. 620-625 32. Identify, outline and assess the value of 32. Use diagrams of GLC and HPLC equipment to the following techniques in the analysis of explain how these techniques can be used to small samples: (a) gas-liquid analyse small samples of forensic materials. chromatography (b) high performance liquid chromatography
5 6 Sensitive Analytical techniques Sensitive Analytical Techniques Chapter 20 33. Outline how a mass spectrometer operates 33. Use a diagram of a mass spectrometer to and clarify its use for forensic chemists explain how it operates. Explain how it can be used 33. 626- 629 34. Analyse and present information from in forensic analysis. secondary sources to discuss the ways in which 34. Use secondary sources to analyse examples of 34. 629-633 analytical techniques may provide evidence how analytical techniques can be used by forensic about samples chemists. Revision Questions - Jacaranda Chemistry 2. Set Revision 20.2 20.2 Pages 633-637
Emission spectra and forensic chemistry Emission spectra and forensic chemistry
35. Describe the conditions under which atoms 35. Demonstrate the emission of light when salts will emit light are vapourised in a Bunsen flame. Describe the 35. 637- 639 36. Identify that the emission of quanta of conditions that lead to light emission. energy as electrons move to lower energy levels 36/37 639- 641 may be detected by humans as a specific colour 36. Identify using diagrams that electrons exist in 37. Explain why excited atoms in the gas phase quantised energy levels. Explain how particular 38. 641 emit or absorb only certain wavelengths of light frequencies of light are related to quantised energy 38. Account for the fact that each element gaps using Planck's equation. produces its signature line emission spectrum 37. Use Bohr's theory to explain how light photons can be emitted from excited atoms. 38. Use energy level diagrams to explain the unique nature of the spectra of each element.
6 7 Emission spectra and forensic chemistry Emission spectra and forensic chemistry Chapter 20 39. Discuss the use of line emission spectra to 39. Describe the principle of atomic emission 39. 641- 644 identify the presence of elements in chemicals spectroscopy and explain how it is used to identify 40. Identify data, choose equipment, plan, and elements in chemicals. perform a first-hand investigation using flame tests and/or spectroscope analysis as appropriate 40. PRACTICAL ACTIVITY 20.1 - Observing 40. 650 - 651 to identify and gather first-hand information to emission spectra describe the emission spectrum of a range of elements including Na and Hg 41. Process and present information from 41. DATA ANALYSIS 20.2 - Identifying 41. 644 secondary sources to analyse and identify elements using emission spectra 652 individual elements present in a mixed emission spectrum and use available evidence to explain how such information Revision Questions - Jacaranda Chemistry 2. Set Revision 20.3 can assist analysis of the origins of a 20.3 Pages 644-647 mixture 8 GENERAL REVISION CD- MODULE Option 3 - REVISION CD Option Module QUESTIONS - Chapters 19 and 20 3 - REVISION QUESTIONS Use the supplied set of questions to revise all of Option Module 3
20 Multiple Choice and 20 open-ended questions in the style of the HSC are available.
Model answers are available on the CD.
7