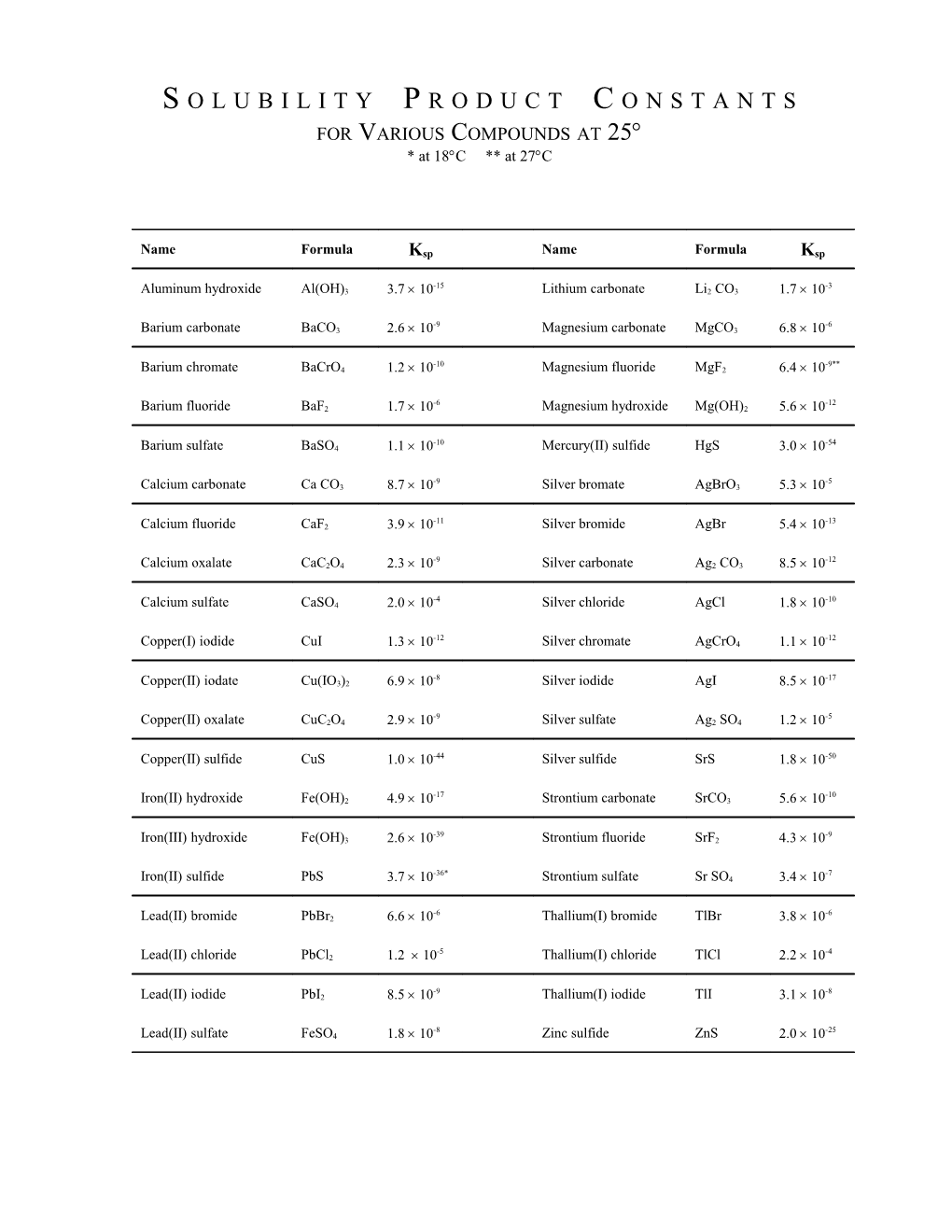
S O L U B I L I T Y P R O D U C T C O N S T A N T S FOR VARIOUS COMPOUNDS AT 25 * at 18C ** at 27C
Name Formula Ksp Name Formula Ksp
-15 -3 Aluminum hydroxide Al(OH)3 3.7 10 Lithium carbonate Li2 CO3 1.7 10
-9 -6 Barium carbonate BaCO3 2.6 10 Magnesium carbonate MgCO3 6.8 10
-10 -9** Barium chromate BaCrO4 1.2 10 Magnesium fluoride MgF2 6.4 10
-6 -12 Barium fluoride BaF2 1.7 10 Magnesium hydroxide Mg(OH)2 5.6 10
-10 -54 Barium sulfate BaSO4 1.1 10 Mercury(II) sulfide HgS 3.0 10
-9 -5 Calcium carbonate Ca CO3 8.7 10 Silver bromate AgBrO3 5.3 10
-11 -13 Calcium fluoride CaF2 3.9 10 Silver bromide AgBr 5.4 10
-9 -12 Calcium oxalate CaC2O4 2.3 10 Silver carbonate Ag2 CO3 8.5 10
-4 -10 Calcium sulfate CaSO4 2.0 10 Silver chloride AgCl 1.8 10
-12 -12 Copper(I) iodide CuI 1.3 10 Silver chromate AgCrO4 1.1 10
-8 -17 Copper(II) iodate Cu(IO3)2 6.9 10 Silver iodide AgI 8.5 10
-9 -5 Copper(II) oxalate CuC2O4 2.9 10 Silver sulfate Ag2 SO4 1.2 10
Copper(II) sulfide CuS 1.0 10-44 Silver sulfide SrS 1.8 10-50
-17 -10 Iron(II) hydroxide Fe(OH)2 4.9 10 Strontium carbonate SrCO3 5.6 10
-39 -9 Iron(III) hydroxide Fe(OH)3 2.6 10 Strontium fluoride SrF2 4.3 10
-36* -7 Iron(II) sulfide PbS 3.7 10 Strontium sulfate Sr SO4 3.4 10
-6 -6 Lead(II) bromide PbBr2 6.6 10 Thallium(I) bromide TlBr 3.8 10
-5 -4 Lead(II) chloride PbCl2 1.2 10 Thallium(I) chloride TlCl 2.2 10
-9 -8 Lead(II) iodide PbI2 8.5 10 Thallium(I) iodide TlI 3.1 10
-8 -25 Lead(II) sulfate FeSO4 1.8 10 Zinc sulfide ZnS 2.0 10