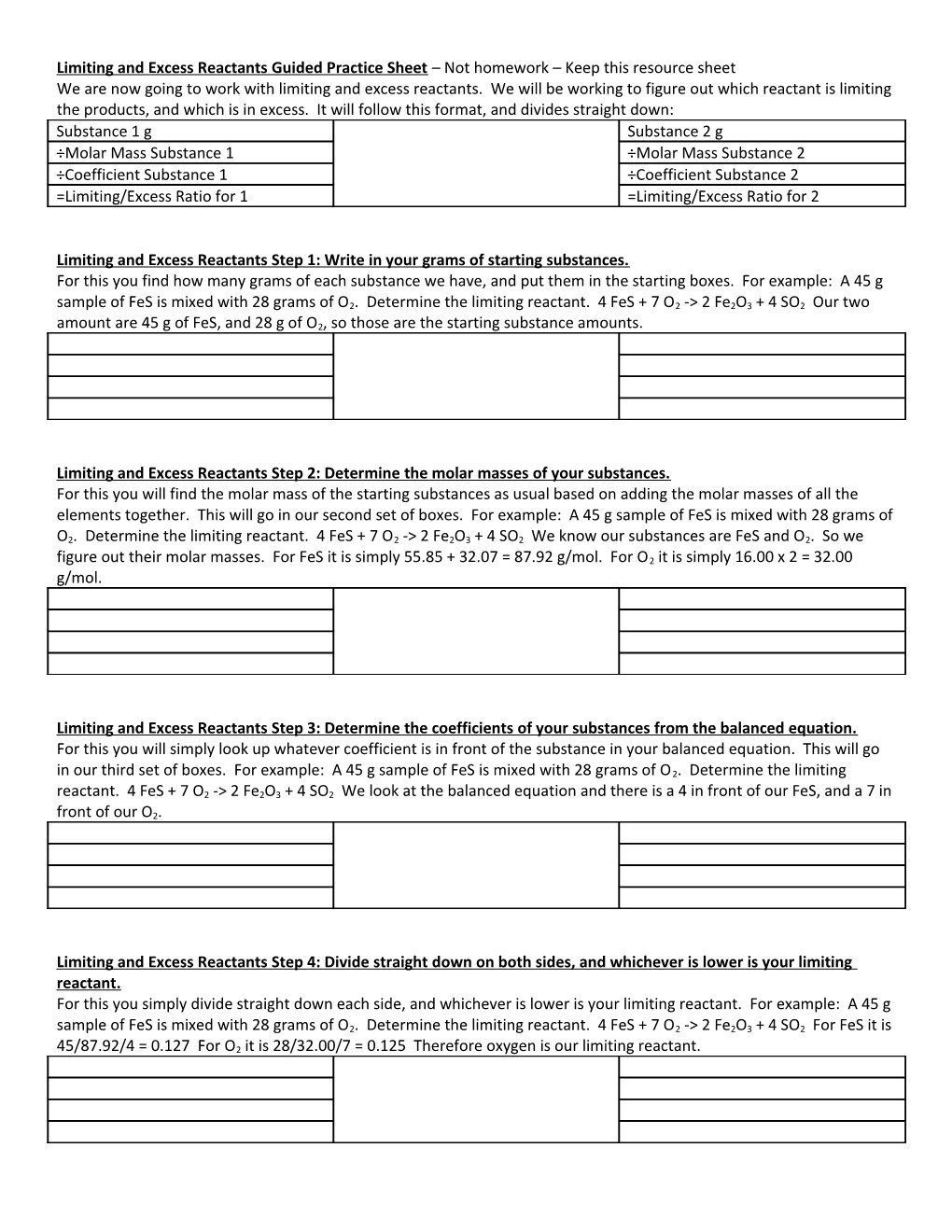
Limiting and Excess Reactants Guided Practice Sheet – Not homework – Keep this resource sheet We are now going to work with limiting and excess reactants. We will be working to figure out which reactant is limiting the products, and which is in excess. It will follow this format, and divides straight down: Substance 1 g Substance 2 g ÷Molar Mass Substance 1 ÷Molar Mass Substance 2 ÷Coefficient Substance 1 ÷Coefficient Substance 2 =Limiting/Excess Ratio for 1 =Limiting/Excess Ratio for 2
Limiting and Excess Reactants Step 1: Write in your grams of starting substances. For this you find how many grams of each substance we have, and put them in the starting boxes. For example: A 45 g sample of FeS is mixed with 28 grams of O2. Determine the limiting reactant. 4 FeS + 7 O2 -> 2 Fe2O3 + 4 SO2 Our two amount are 45 g of FeS, and 28 g of O2, so those are the starting substance amounts.
Limiting and Excess Reactants Step 2: Determine the molar masses of your substances. For this you will find the molar mass of the starting substances as usual based on adding the molar masses of all the elements together. This will go in our second set of boxes. For example: A 45 g sample of FeS is mixed with 28 grams of
O2. Determine the limiting reactant. 4 FeS + 7 O2 -> 2 Fe2O3 + 4 SO2 We know our substances are FeS and O2. So we figure out their molar masses. For FeS it is simply 55.85 + 32.07 = 87.92 g/mol. For O2 it is simply 16.00 x 2 = 32.00 g/mol.
Limiting and Excess Reactants Step 3: Determine the coefficients of your substances from the balanced equation. For this you will simply look up whatever coefficient is in front of the substance in your balanced equation. This will go in our third set of boxes. For example: A 45 g sample of FeS is mixed with 28 grams of O2. Determine the limiting reactant. 4 FeS + 7 O2 -> 2 Fe2O3 + 4 SO2 We look at the balanced equation and there is a 4 in front of our FeS, and a 7 in front of our O2.
Limiting and Excess Reactants Step 4: Divide straight down on both sides, and whichever is lower is your limiting reactant. For this you simply divide straight down each side, and whichever is lower is your limiting reactant. For example: A 45 g sample of FeS is mixed with 28 grams of O2. Determine the limiting reactant. 4 FeS + 7 O2 -> 2 Fe2O3 + 4 SO2 For FeS it is
45/87.92/4 = 0.127 For O2 it is 28/32.00/7 = 0.125 Therefore oxygen is our limiting reactant. Examples:
A 37 g sample of AgI is mixed with 64 grams of Na2S. Determine the limiting reactant. 2 AgI + Na2S -> Ag2S + 2 NaI
A 186 g sample of Na3PO4 is mixed with 97 grams of HCl. Determine the limiting reactant. Na3PO4 + 3 HCl -> 3 NaCl +
H3PO4
Now you try:
A 57 g sample of NaBr is mixed with 153 grams of Cl2. Determine the limiting reactant. 2 NaBr + Cl2 -> 2 NaCl + Br2
_____ g NaBr _____ g Cl2 ÷_____ g/mol ÷_____ g/mol
÷_____ coefficient of NaBr ÷_____ coefficient of Cl2
=_____ limiting ratio of NaBr =_____ limiting ratio of Cl2
A 36 g sample of CaCl2 is mixed with 196 grams of Na3PO4. Determine the limiting reactant. 3 CaCl2 + 2 Na3PO4 ->
Ca3(PO4)2 + 6 NaCl
A 50 g sample of PCl5 is mixed with 150 grams of H2O. Determine the limiting reactant. PCl5 + 4 H2O -> H3PO4 + 5 HCl