Appendix (To be placed on the EMS of the journal)
THE KINETIC PARTICLE THEORY DIAGNOSTICIC TEST
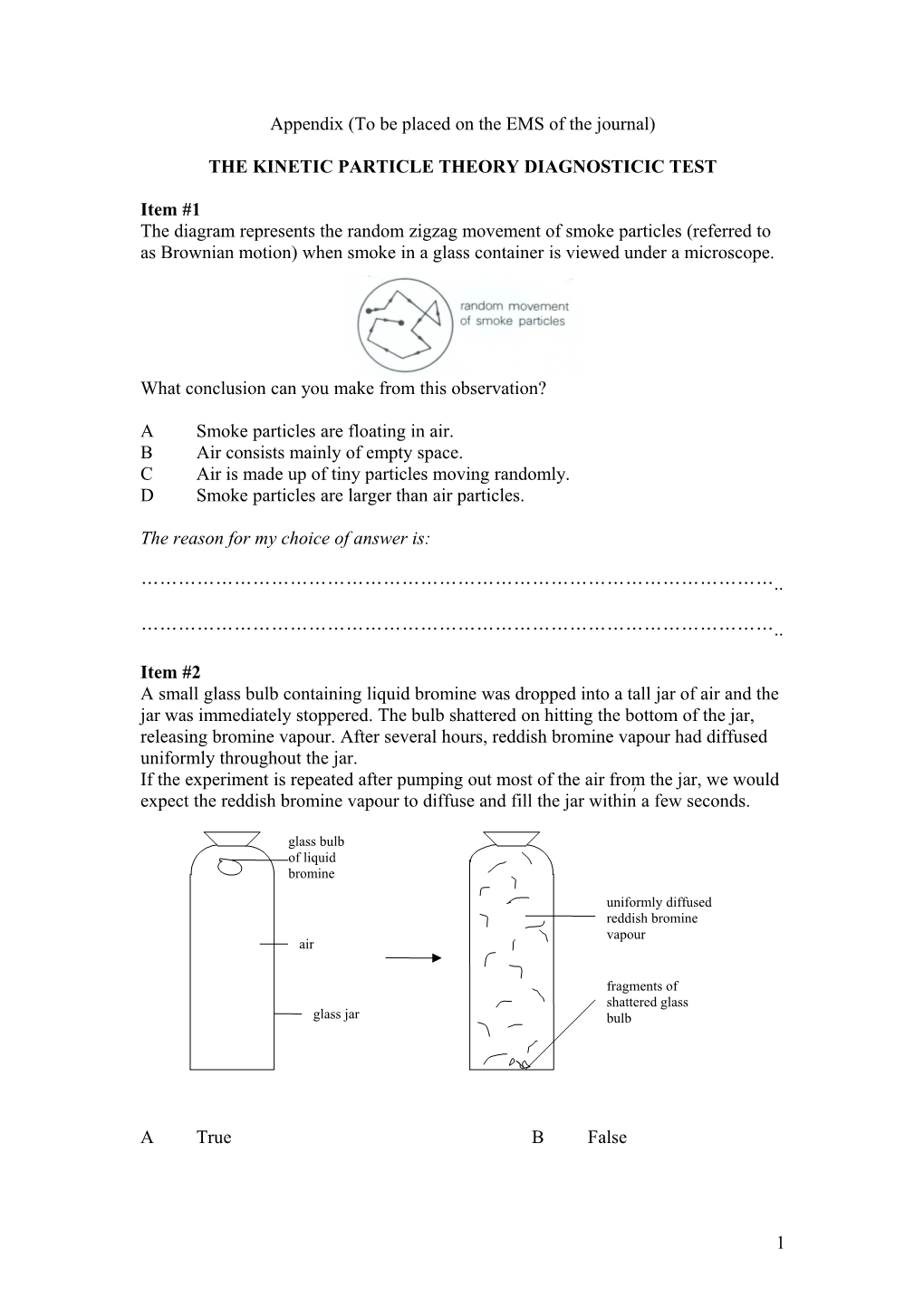
Item #1 The diagram represents the random zigzag movement of smoke particles (referred to as Brownian motion) when smoke in a glass container is viewed under a microscope.
What conclusion can you make from this observation?
A Smoke particles are floating in air. B Air consists mainly of empty space. C Air is made up of tiny particles moving randomly. D Smoke particles are larger than air particles.
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #2 A small glass bulb containing liquid bromine was dropped into a tall jar of air and the jar was immediately stoppered. The bulb shattered on hitting the bottom of the jar, releasing bromine vapour. After several hours, reddish bromine vapour had diffused uniformly throughout the jar. If the experiment is repeated after pumping out most of the air from the jar, we would expect the reddish bromine vapour to diffuse and fill the jar within a few seconds.
glass bulb of liquid bromine
uniformly diffused reddish bromine vapour air
fragments of shattered glass glass jar bulb
A True B False
1 The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #3 When lemonade from a soft drink can is poured into a tall glass, the volume of lemonade increases.
A True B False
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #4 The diagram shows a coloured gas being compressed in a gas syringe until the plunger could not be pushed any further. The experiment was repeated using the same volume of a coloured liquid.
It was found that the final volume of the gas was
A much less than that of the liquid. B much greater than that of the liquid.
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
2 Item #5 The diagram shows a pump containing a coloured gas that is compressed by pushing the plunger down.
We can conclude that
A the volume and mass of air in the pump have decreased. B the volume of air has decreased while the mass has increased. C the volume of air has decreased while the mass remains constant.
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #6 A small amount of blue ink was carefully placed at the bottom of a test-tube containing some water as shown in the diagram.
After several days, the ink would have diffused throughout the water producing a uniformly blue solution.
A True B False The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
3 Item #7 A balloon is inflated and tied at the neck to prevent it from deflating. The diagram shows a magnified view of the skin of the balloon and the particles in the inflated balloon.
magnified view of balloon skin
molecules that have escaped through the skin of the balloon
molecules bouncing off the inside of the skin of the balloon After several hours, the balloon would be found to remain the same size. A True B False
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #8 The diagram shows how the temperature changes when a solid like naphthalene is heated gently until it melts. In which section of the curve is the heat energy that is absorbed not heating up the naphthalene?
A (a) B (b) C (c)
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
4 Item #9 The diagram shows how the temperature changes when some ice at a temperature below 0oC is heated to above 100oC.
We can conclude from the graph that that liquid water cannot exist at its boiling point of 100oC.
A True B False
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
Item #10 The diagram shows the arrangement of particles in different states of matter. e r u t a r e p e m r e t u
t r a e r h
e boiling condensing g p i h m e t
r
e boiling condensing h g i h
melting freezing
melting freezing
In which of the changes of state will heat energy be absorbed? A solid → liquid → gas B gas → liquid → solid The reason for my choice of answer is:
…………………………………………………………………………………………..
5 ………………………………………………………………………………………….. Item #11 The diagram shows that the total volume of liquid decreases when water and alcohol are mixed together.
We can conclude that some of the alcohol has evaporated.
A True B False
The reason for my choice of answer is:
…………………………………………………………………………………………..
…………………………………………………………………………………………..
…………………………………………………………………………………………..
6 Figure A No. Propositional content knowledge statements
S1 All matter is made of small particles. S2 The particles in a solid may be arranged in a regular pattern and are continuously vibrating about their fixed positions. S3 The particles in a liquid are in slow random motion within the volume of the liquid, continually colliding with each other. S4 The particles in a gas move about freely in rapid random motion, continually colliding with each other. S5 The particles in solids are very close together, generally slightly further apart in liquids and much more widely spaced in gases. S6 The forces of attraction between the particles are very strong in solids, weaker in liquids and almost negligible in gases. S7 Solids have a definite shape and volume because their particles occupy fixed positions. S8 Liquids take the shape of the container because their particles are able to move about within a fixed volume. S9 Liquids have a definite volume because their particles are attracted to one another at that temperature. S10 Gases do not have a fixed shape or volume and occupy the entire space that is available because of the very weak forces of attraction between their particles. S11 Solids and liquids are difficult to compress because their particles are close together. S12 Gases can be readily compressed because their particles are widely spaced. S13 Particles in liquids are able to diffuse because of the weaker forces of attraction between their particles compared to those in solids. S14 Particles in gases diffuse readily because of the very weak forces of attraction between the particles. S15 During melting of a solid, the particles that are in fixed positions gain sufficient energy to overcome the attractive forces between the particles and move about in the liquid state. S16 During evaporation or boiling of a liquid, the particles gain enough energy to weaken the attractive forces between the particles and move about freely in the vapour or gaseous state. S17 The kinetic energy of particles increases as heat in added. S18 The temperature remains constant at the melting point and the boiling point of a substance until sufficient heat energy has been absorbed to weaken the forces of attraction between the particles in the solid and liquid states, respectively. Figure A. Propositional content knowledge statements about the kinetic particle theory of matter.
7 TABLE A
Difficulty and discrimination indices for the 12 items in the KPTI Item Difficulty Discrimination Item Difficulty Discrimination no. index index no. index index 1 0.8 0.38 7 0.9 0.10 2 0.6 0.43 8 0.9 0.20 3 0.9 0.18 9 0.9 0.23 4 0.9 0.20 10 0.9 0.28 5 0.9 0.35 11 0.8 0.40 6 0.8 0.40
Notes:
Difficulty (or facility) indices for the combined sample ranged from 0.6 to 0.9.
Only Item 2 was of moderate difficulty (in the range 0.4 – 0.6), while all the remaining 10 items were considered easy with difficulty indices greater than 0.6.
Ideally an item should be of moderate difficulty with a difficulty index between 0.4 and 0.6. The discrimination indices for the 11 items ranged from 0.10 to 0.43. Items that have discrimination indices greater than 0.40 discriminate well between low and high achievement students. Items with discrimination indices between 0.20 and 0.40
(Items 1, 4, 5, 6, 8, 9, 10 and 11) are satisfactory in this respect. Hence, although 10 of the items were considered easy, eight of these items discriminated satisfactorily between the high achieving and low achieving students in the sample.
TABLE B
8 Percentage of students’ who correctly answered all multiple-choice items and provided scientifically acceptable explanations relating to (1) intermolecular spacings in solids, liquids and gases, (2) effect of intermolecular forces on changes of state, and (3) diffusion in liquids and gases (the numbers of students are denoted in parentheses)
Conceptual Hong Kong Singapore Brunei Australia Combined categories (Grade 10) (Grade 10) (Grade 10) (Grade 9) sample (N = 37) (N = 66) (N = 24) (N = 21) (N = 148) (1) Items 3, 4, 0 (0) 7.6 (5) 0 (0) 0 (0) 3.4 (5) 5 & 11
(2) Items 8, 9 2.7 (1) 28.8 (19) 0 (0) 0 (0) 13.5 (20) & 10
(3) Items 1, 2, 0 (0) 1.5 (1) 0 (0) 0 (0) 0.7 (1) 6 & 7
Notes:
Consistency in students understanding of the intermolecular spacing in solids, liquids and gases (Items 3, 4, 5 & 11). Correct responses to all four multiple-choice items relating to the intermolecular spacing in the three states of matter were provided by 92 students (62.2%) (see Table V of the manuscript). However, only five students were able to provide scientifically acceptable justifications for selecting the correct responses to all five items.
Consistency in students’ understanding of the effects of intermolecular forces on changes of state (Items 8, 9 & 10). Correct responses to all three items relating to the effects of intermolecular forces on state changes were provided by 116 students
(78.4%) (see Table V of the manuscript). However, only 20 of these students provided scientifically acceptable justifications for selecting the correct responses to all three items.
9 Consistency in students’ understanding of diffusion in liquids and gases in terms of the particulate nature of matter (Items 1, 2, 6 & 7). Although 60 students (40.5%) chose the correct responses to all four items relating to diffusion in liquids and gases
(see Table V of the manuscript), only one student was able to provide scientifically acceptable justifications for the correct responses to all these four items.
10