BIOTECHNOLOGY SYLLABUS
SCHEME OF INSTRUCTION AND EXAMINATION
B.Tech. (Biotech) - IV YEAR - II Semester
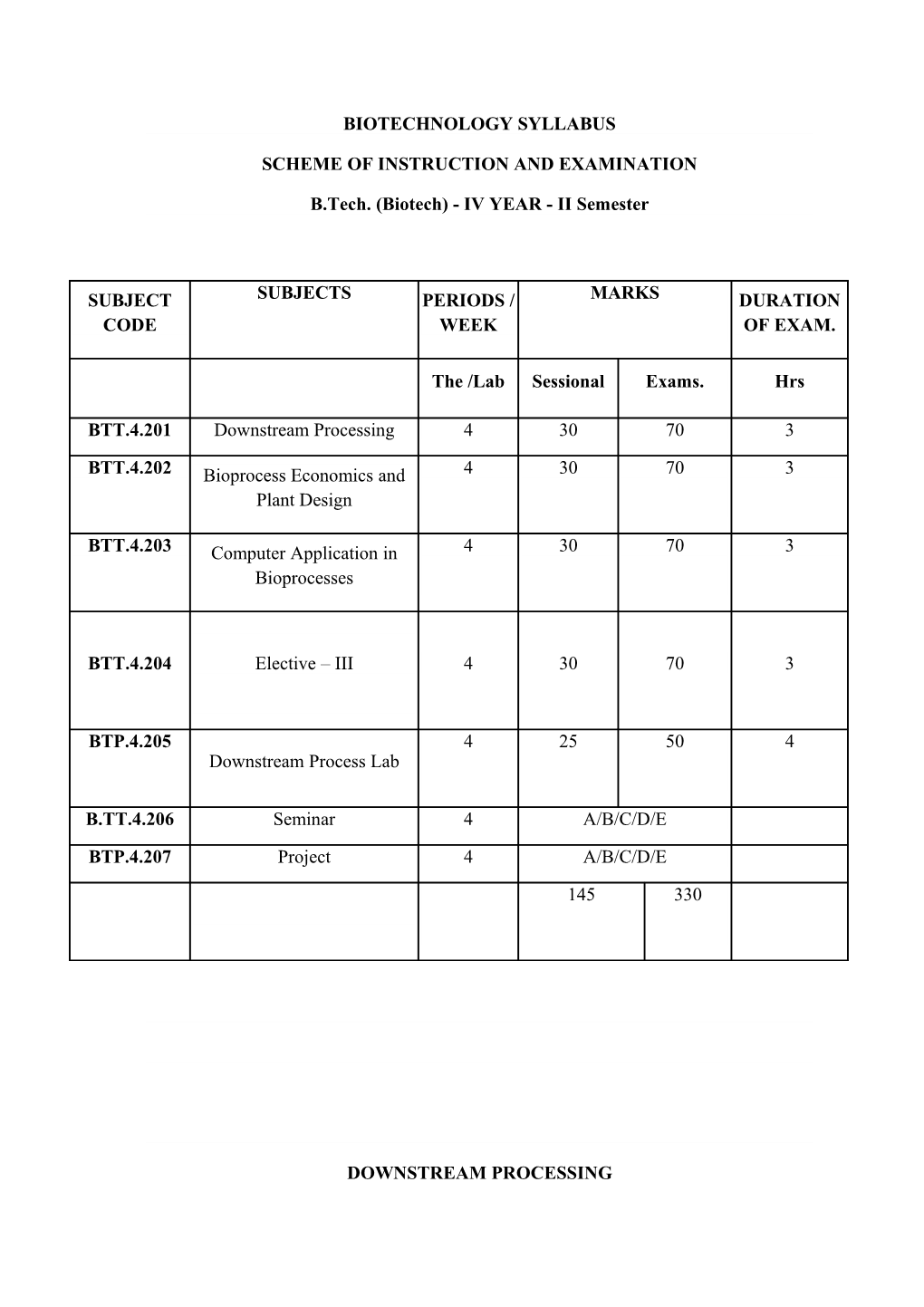
SUBJECT SUBJECTS PERIODS / MARKS DURATION CODE WEEK OF EXAM.
The /Lab Sessional Exams. Hrs
BTT.4.201 Downstream Processing 4 30 70 3
BTT.4.202 Bioprocess Economics and 4 30 70 3 Plant Design
BTT.4.203 Computer Application in 4 30 70 3 Bioprocesses
BTT.4.204 Elective – III 4 30 70 3
BTP.4.205 4 25 50 4 Downstream Process Lab
B.TT.4.206 Seminar 4 A/B/C/D/E
BTP.4.207 Project 4 A/B/C/D/E
145 330
DOWNSTREAM PROCESSING UNIT- I ROLE OF DOWNSTREAM PROCESSING IN BIOTECHNOLOGY Subject code : BTT.4.201 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
Role and Importance of Downstream Processing in Biotechnological Processes; Characterization of Biomolecules and fermentation broths; Physico-Chemical basis of Bio-separations; Characteristics of Bio-separations; Broad Strategies for design of Bio- separation processes. Recent developments in product isolation.
UNIT- II PRIMARY SEPARATION AND RECOVERY PROCESSES
Cell Disruption methods for intracellular products- Mechanical and Chemical Methods; Removal of Insolubles, biomass (and Particulate debris) separation techniques; Flocculation and Sedimentation; Centrifugation and Filtration methods.
UNIT- III PRODUCT ENRICHMENT OPERATIONS
Membrane-based separations- Micro-filtration, Ultra-filtration, Dialysis, Electro dialysis, Reverse Osmosis; Theory, design and configuration of membrane separation equipment, Applications; Aqueous Two-phase extraction of proteins; Precipitation of proteins with salts and organic solvents; Adsorption processes.
UNIT- IV PRODUCT PURIFICATION AND POLISHING
Chromatographic separations- Principles, Elution Chromatography, IMAC, Bio-affinity Chromatography; Design and selection of chromatographic matrices; Design of large- scale chromatographic separation processes.
UNIT- V- NEW AND EMERGING TECHNOLOGIES: Dialysis, Crystallization Pervaporation, super liquid extraction foam based separation with examples for processing of Two Industrial Products (Citric acid / Penicillin and Low volume high value product like recombinant proteins). Electrophoretic Separations; Final Product Polishing-Crystallization, Drying, Lyophilization; Formulation Strategies.
EXAMINATION: One question from each unit with internal choice TEXT BOOKS:
1. Bio-separations: Principles And Techniques (2008) Prentice-hall Of India Pvt Ltd 2. Separation processes in Biotechnology by Sivasankar B, J M Asenjo, Marcel- Dekker, (1993). 3. Bio-separations- Downstream Processing for Biotechnology- Paul A Belter, E L Cussler, Wei-shouHu, Wiley Inter-science Publications, 1988. 4. Principles and Techniques of Practical Biochemistry by Keith Wilson, John Walker, John M. Walker 5th edition Cambridge University Press, (2000).
REFERENCE BOOKS:
1. Product Recovery in Bioprocess Technology- BIOTOL series, Butterworth Heinmann, (1992).
2. Separations for Biotechnology- M S Verall, M J Hudson, Ellis Harwood Ltd. (1990).
3. Bio-separations Science and technology Roger Todd Rudge Petreides Process Biotechnology Fundamentals by SN Mukhopadhya, Wankat PC. Rate controlled separations, Elsevier, (1990).
4. Bioseparations by Belter PA and Cussler E., Wiley (1985).
5. Product Recovery in Bioprocess Technology, BIOTOL.’ Series, VCH, (1990).
6. Separation processes in Biotechnology Asenjo J.M., (1993), Marcel Dekkere Inc.
7. Bioseparations by Siva Shankar PHI publications. BIOPROCESS ECONOMICS & PLANT DESIGN
Subject code : BTT.4.202 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
UNIT-I ECONOMIC EVALUATION
Capital cost of a project; Interest calculations, nominal and effective interest rates; basic concepts in tax and depreciation; Measures of economic performance, rate of return, payout time; Cash flow diagrams; Cost accounting-balance sheet and profit loss account; Break even and minimum cost analysis.
UNIT- II BIOPROCESS ECONOMICS
Bio-Products regulations; Economic analysis of bioprocess; Capital, overhead and manufacturing costs estimation; Case studies of antibiotics, recombinant products, single cell protein, anaerobic processes and other fine chemicals.
UNIT- III INTRODUCTION TO PLANT DESIGN
Process design development: design procedures, design information and flow diagrams, material and energy balances, comparison of different process and design specifications; Optimization; General design considerations: Health and safety hazards, Environment protection, plant location and plant layout, plant operation and control;
UNIT- IV BASIC DESIGN PROBLEMS
Design examples on continuous fermentation, aeration, and agitation; Design calculation of filter for air sterilization; Design of batch and continuous sterilizers; Design calculations for immobilized enzyme kinetics; Practical considerations in designing of Bioreactor/Fermentor construction. UNIT- V
Introduction to different types of valves, pumps, steam traps, spargers and impellers used in fermentation industries; Design exercise on trickle flow fermentor; Problems associated with design equations.
EXAMINATION: One question from each unit with internal choice
TEXT BOOKS
1. Plant Design and Economics for Chemical Engineers, 5/e Max S. Peters, Ronald E. West , (2003) McGraw-Hill Higher,
2. Biochemical Engineering - Humphrey, A. E.; Millis, JSTOR 1966. 3. Biochemical Engineering, by Harvey W. Blanch, Douglas S. Clark CRC; 1st edition (1997). 4. Biochemical Engineering Fundamentals by James; Ollis, David F. Bailey,1977, McGraw-Hill.
REFERENCE BOOKS:
1. Biochemical Engineering and Biotechnology Handbook by Bernard Atkinson, Ferda Mavituna Grove's Dictionaries; 2 edition (1992).
2. Bioprocess Engineering:Basic Concepts. Michael L. Shuler / Fikret Kargi, Reihe:Prentice ,(2001) Hall.
3. Plant Design and Economics for Chemical Engineers” by M. Peters and K. Timmerhaus, McGraw-Hill.
4. Bioprocess and Biosystems Engineering Dirk Weuster-Botz, ISSN: 1615-7591 Journal no. 449, Springer.
5. Putting Biotechnology to Work: Bioprocess Engineering ISBN-10: 0-309-04785-4, National Academy Of Sciences Washington. Committee on Bioprocess Engineering Board on Biology Commission on Life Sciences.
6. Chemical Engineering plant Design by C.Vilbrandt and Dryden C.E. 4th Edition Mc Graw Hill Book Co.
7. Process Engineering Economics. by H.E. Schweyer, Mc Graw Hill Co. COMPUTER APPLICATIONS IN BIOPROCESS INDUSTRY
Subject code : BTT.4.203 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
The Programs are to be written in "C" only
UNIT-I
Computers and Software: Computing environments, The software development processes, Algorithm design, Program composition, Quality Control, Documentation, Storage and •Maintenance, Software strategy. Process Models: Uses, Distributed & Lumped parameter models, Linear and Nonlinear models, Steady state and Dynamic models, Continuous and Discrete models, Empirical models. Formulation of Process Models: Momentum, mass and energy balances, constitutive rate equations, transport rate equations, chemical kinetic rate expressions, thermodynamic relations.
Review on "C" Language Fundamentals.
UNIT-II
Formulation Process Models leading to set of ordinary differential equations and solution procedures by Eulers, Modified Eulers and Runge Kutta methods.
UNIT-III
Formulation of Process Models leading to set of linear simultaneous equations and solution procedures by Method of determinants, Gauss Elimination, Gauss Jordan, Jacobi and Gauss-Seidel methods.
UNIT-IV
Formulation of Process Models leading to transcendental and polynomial equations and solution procedures by Bi-section, Reguli-falsi, Newton Raphson, Richmond, Muller's and Bairstow methods UNIT-V
Function Approximations by linear and nonlinear least square analysis, Approximations by orthogonal functions, chebyshev polynomials.
EXAMINATION: One question from each unit with internal choice
SUGGESTED BOOKS:
1. Higher engineering mathematics by DR. B.S. Grewal, Khanna publishers (1998) 2. Numerical methods for Engineers by Steven C. Chapra and Raymond P Canale, 2nd edition, MCGraw Hill International edition, 1988. 3. Computer Applications in Bioprocessing by Henry R. Bungay Volume 70/(2000) Springer. 4. Bioprocess engineering Enrique Galindo and Octavio T. Ramírez Volume 16, Issue 7, (1998). 5. Computer Applications In Biotechnology (2004) By Marie-Noelle Pons and Jan Van Impe (2005), ELSEVIER. ELECTIVE- III
Subject code : BTT.4.204 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
BIOSENSORS & BIOELECTRONICS
UNIT- I: INTRODUCTION Introduction to Biosensors- Advantages and Their Limitations, Various components; Biocatalysts based biosensors, Bio-affinity based biosensors and Microorganisms based biosensors; biologically active material and analysis; Types of membranes used in biosensor constructions.
UNIT- II: TRANSDUCERS IN BIOSENSORS Various types of transducers; Principles and applications- Colorimetric, Optical, Potentiometric, Amperometric, Conductometric, Resistometric, Piezoelectric, Semiconductor, Impedimetric, Mechanical and Molecular electronic based transducers. Chemiluminiscence based biosensors.
UNIT- III: APPLICATIONS OF BIOSENSORS Biosensors in clinical chemistry, medicine and health care; Biosensors for veterinary, agriculture and food; Low cost biosensors for industrial processes for online monitoring; Biosensors for environmental monitoring.
UNIT- IV: MOLECULAR ELECTRONICS Potential advantages and development towards a bimolecular computer; Development of Molecular arrays as a memory stores; Molecular wires and switches; Mechanisms of Unit assembly.
UNIT- V: DESIGN FOR A BIOMOLECULAR PHOTONIC COMPUTER Assembly of photonic bimolecular memory store; Information Processing; Commercial prospects for bimolecular computing systems. EXAMINATION: One question from each unit with internal choice
TEXT BOOKS: 1. Biosensors Elizebeth A.H.Hall Open Univ Press Milton Raynes Commercial. 2. Biosensors: Applications to Clinical, Bioprocess, and Environmental Samples Wiley-Interscience; 1St edition (1998).
REFERENCE BOOKS: 1. Biosensors: An Introduction by Brian R. Eggins Biosensors edited by AEG CASS OIRL press Oxford University John Wiley & Sons (1997). 2. Transducers and instrumentation by Murthy DVS Prentice Hall 1995. 3. An Introduction to Systems Biology: Design Principles of Biological Circuits by Uri Alon , Chapman & Hall/CRC; 1st edition, (2006).
Subject code : BTT.4.204 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
ELECTIVE- III
MOLECULAR MODELING & DRUG DESIGN
UNIT- I EMPERICAL FORCE FIELDS AND MOLECULAR MECHANISMS
Bond Stretching- Angle bending- Torsional I terms; Out of plane; Bonding Motions; Electrostatic interactions; Vander Walls interactions; Effective pair potentials; Hydrogen Bonding; Simulation of liquid water.
UNIT- II COMPUTER SIMULATION METHODS
Calculation of thermodynamic properties; Phase space; Practical aspects of computer simulation; Boundaries monitoring Equilibrium; Long range process; Analyzing results of simulation and estimating errors.
UNIT- III MOLECULAR DYNAMICS SIMULATION METHODS
Molecular Dynamics using simple modules; Molecular Dynamics with continuous potentials; Running Molecular Dynamics Simulation; Constant Dynamics; Time dependent properties; Molecular Dynamics at constant temperature and pressure.
UNIT- IV MONTECARLO SIMULATION METHODS
Metropolis methods; Monte Carlo simulation of molecules; Monte Carlo simulation of Polymers; Calculating Chemical potentials; Monte Carlo simulation of molecular dynamics. UNIT V- APPLICATIONS OF MOLECULAR MODELING IN DRUG DESIGN
Quantitative structural activity relationship (QSAR) studies in Protein-Ligand interactions. Case studies of Alzheimer’s disease, tuberculosis and cancer etc.
EXAMINATION: One question from each unit with internal choice
TEXT BOOKS:
1. Molecular Modeling Principles and Applications- AR Leach, Longman, (1996). 2. Molecular Dynamics Simulation- Elementary Methods- John Wiley and Sons, (1997). REFERENCE BOOKS:
1. Protein Engineering - Moody PCE and AJ Wilkinson. IRL press. 2. Introduction to Protein Structure by C. Brandon and J. Tooze, Garland, 2nd edition, (1998). ELECTIVE- III
CREATIVITY, INNOVATION & NEW PRODUCT DEVLOPMENT
Subject code : BTT.4.204 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
UNIT- I INTRODUCTION
The Process of Technological Innovation. Factors contributing to successful technological innovation, the need for creativity and innovation. Creativity and Problem solving, Brain storming- different techniques.
UNIT- II PROJECT SELECTION AND EVALUATION
Collection of ideas and purpose of project; Selection criteria. Screening ideas for new products. Evaluation Techniques.
UNIT- III NEW PRODUCT DEVELOPMENT AND PLANNING
Research and new product development; Patent- Patent search; Patent laws; International codes for patents; Intellectual Property Rights (IPR).Design of Proto type, Testing, Quality standards; Marketing research; Introducing new concepts-GMP.
UNIT- IV LABORATORY
Creative Design, Model Preparation, Testing; Cost evaluation; Patent application; Good Laboratory Practice (GLP).
UNIT V: Product Deployment and Commercialization-Case Studies
EXAMINATION: One question from each unit with internal choice TEXT BOOKS:
1. Harry b.watton - New product planning. Prentice-hall inc. (1992).
2. P.N.khandwalla - Fourth eye (excellence through creativity) – wheeler Publishing, Allahbad, (1992).
REFERENCE BOOKS:
1. Harry Nystrom - Creativity and innovation -John Wiley & sons, 1979.
2. Managing technological innovation, Brain Twiss, pitman publishing ltd, (1992).
3. I.P.R. bulletins. Tifac, New Delhi, 1997.
4. Creativity and innovation- Harry Nystrom, John wiley & sons, (1979).
5. Managing technological innovation- brain twiss, Pitman publishing ltd., (1992).
6. New product planning- Watton HB, Prentice-hall inc., (1992).
7. Fourth eye (excellence through creativity)- Khandwalla PN Wheeler publishing, (1992). ELECTIVE- III
IMMUNODIAGNOSTICS
Subject code : BTT.4.204 Sessional : 30
Periods / Week : 4 Examination : 70
Nature of Examination : Theory Exam Duration : 3 Hrs
UNIT I INTRODUCTION
Principles of immunodiagnostic tests and their development, classification of immunodiagnostic tests, selection and preparation of reagents, Assay design, Antibody engineering, Catalytic antibodies, antibody immunotherapy,
UNIT II HYBRIDOMA TECHNOLOGY
Immunodiagnostics and preparation of tools: Hybridoma technique, monoclonal antibodies production, myeloma cell lines, fusion of myeloma cells, selection of hybridomas, Screening, purification and application (biochemical research, clinical diagnosis and treatment) of monoclonal antibodies.
UNIT III VACCINES
Vaccines and Subunit vaccines-against Herpes Simplex virus, Foot and Mouth disease, live recombinant vaccines-attenuated (Cholera, Salmonella), Vector vaccines directed against viruses and bacteria. Purified vaccines. DNA vaccines. Antifertility vaccines.
UNIT IV NOVEL TECHNIQUES IN IMMUNODIAGNOSTICS
Imaging as an Immunodiagnostic Tool, Multiplex Analysis of Cytokines, Multicolor Flow Cytometry, Immunomonitoring of Clinical Trials, Immunoglobulin and Free-light Chain Detection, Methods for Autoantibody Detection, Immunodiagnostic of Allergy, Immunological Assays Used in Vaccine Clinical Trials
UNIT V IPR ON IMMUNO PRODUCT Transgenic organisms and their uses, Patenting, General Agreement on Trade and Tariff {GATT} and Intellectual Property Rights EXAMINATION: One question from each unit with internal choice
TEXT BOOKS:
1. Immunodiagnostics: a practical approach- By Ray Edwards, Published by Oxford University Press, (1999). 2. Immuno diagnostics by S.C. Rastogi, New Age Publishers. (2001) REFERENCE BOOKS:
1. Kuby Immunology by Thomas J. Kindt (Author), Barbara A. Osborne, Richard Goldsby, W. H. Freeman; 6th edition (2006). 2. Principles of Immunology and Immunodiagnostics by Ralph M. Aloisi Lea & Febiger, (1988). 3. Principles of Immuno pharmacology Nijkamp, Frans P.; Parnham, Michael J. (Eds.)
2nd ed., (2005), ISBN: 978-3-7643-5804-4. A Birkhäuser book. DOWN STREAM PROCESSING LABORATORY
Subject code : BTP.4.205 Sessional : 25
Periods / Week : 4 Examination : 50
Nature of Examination : Theory Exam Duration : 4 Hrs
List of Experiments
1. Cell Disruption by Sonification. 2. Cell Disruption by Enzymatic Reaction. 3. Aqueous Two-phase Extraction. 4. Centrifugal Separation- Ultra Centrifugation, Gel Filtration. 5. Micro filtration. 6. Ultra filtration. 7. Dialysis. 8. Ammonium Sulphate Precipitation. 9. Isoelectric Precipitation. 10. Ion Exchange Chromatography. 11. Affinity Chromatography. 12. High Pressure Liquid Chromatography. 13. Gas Chromatography. 14. Gel Exclusion Chromatography. 15. Crystallization. 16. Freeze Drying. 17. Adsorption Chromatography. 18. Electrophoresis. 19. Extraction of Enzymes. 20. Extraction of RNA and its estimation by Orcinol method. BOOKS:
1. An introduction to Practical Biochemistry III edition by David Plummer John Wiley & Sons. 2. Principles and Techniques of Biochemistry and Molecular Biology by Keith John Walker John Walker, Cambridge University Press; 6 edition (2005). 3. Laboratory Manual in Biochemistry By J. Jayaraman, Kunthala Jayaramanj, New Age International