AROMATIC ANSWERS
1
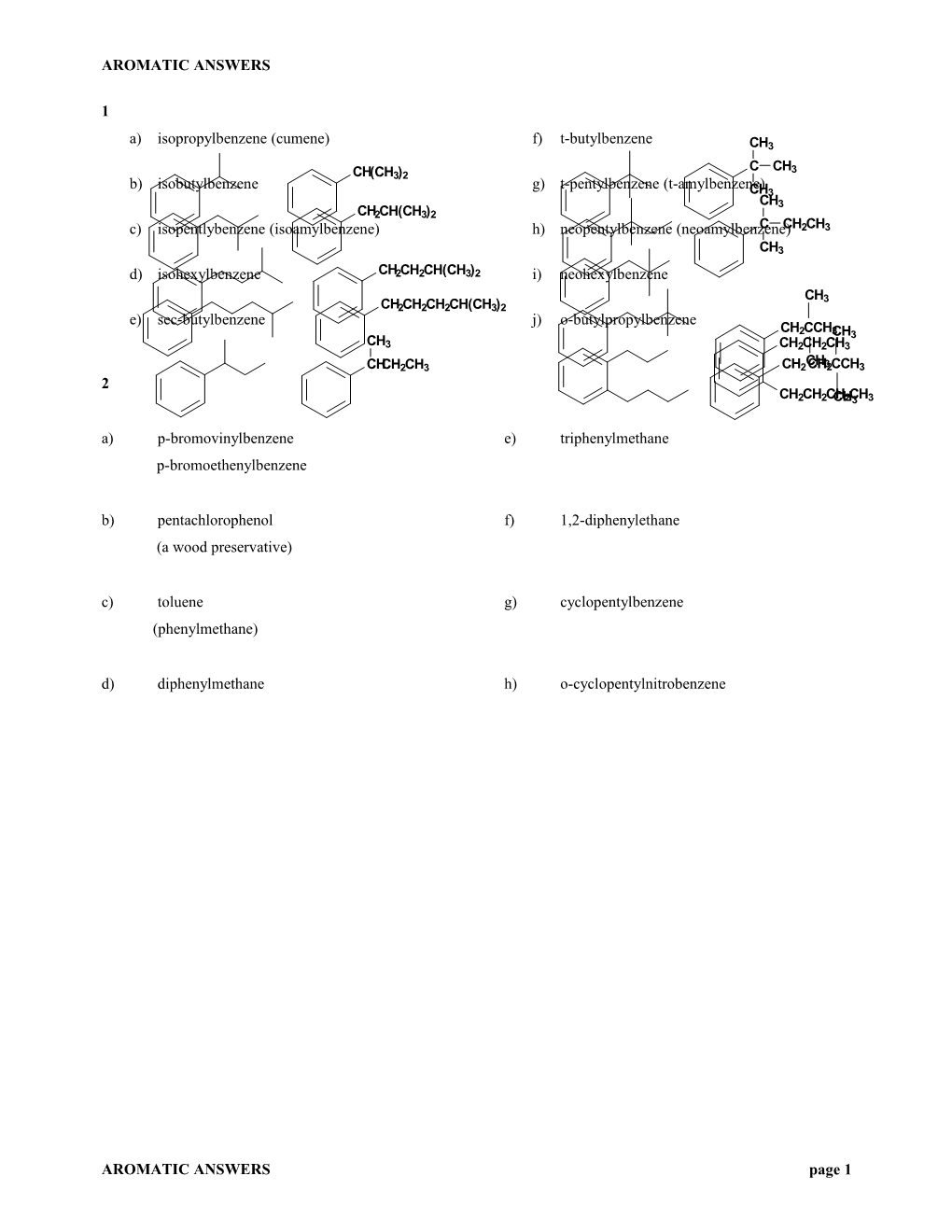
a) isopropylbenzene (cumene) f) t-butylbenzene CH3
C CH3 CH(CH3)2 b) isobutylbenzene g) t-pentylbenzene (t-amylbenzene) CH3 CH3 CH2CH(CH3)2 c) isopentlybenzene (isoamylbenzene) h) neopentylbenzene (neoamylbenzene)C CH2CH3 CH3 d) isohexylbenzene CH2CH2CH(CH3)2 i) neohexylbenzene
CH3 CH2CH2CH2CH(CH3)2 e) sec-butylbenzene j) o-butylpropylbenzene CH2CCH3CH3 CH3 CH2CH2CH3 CH CHCH2CH3 CH2CH32CCH3 2 CH2CH2CCHH2C3H3 a) p-bromovinylbenzene e) triphenylmethane p-bromoethenylbenzene b) pentachlorophenol f) 1,2-diphenylethane (a wood preservative) c) toluene g) cyclopentylbenzene (phenylmethane) d) diphenylmethane h) o-cyclopentylnitrobenzene
AROMATIC ANSWERS page 1 AROMATIC ANSWERS
H H H H CH2
H H H H H H phenyl group benzyl group 3
a) benzyl alcohol f) 4-bromo-3-fluorobenzaldehyde
(phenylmethanol) g) 3-iodobenzyl bromide
b) benzyl chloride or m-(bromomethyl)iodobenzene
(chlorophenylmethane) h) benzophenone
c) 2-hydroxybenzoic acid i) 3-fluorobenzophenone
d) 4-chlorobenzonitrile j) 2-iodoacetophenone
e) 3-nitrobenzoyl chloride
4
a) e) O Br NH2 NH C CH3 3 2 2' 3' b) f) I CH3 1 1' 4 4'
CH3 5 6 6' 5' c) g) H3C CH2 CH3 8 1 Napthalene's tw o quaternary 2 F O 7 C's are not part of the main d) h) numbering since they cannot Cl S OH 6 3 bear any substituents. 58 49 1 5 O Cl Br Cl Cl 7 2
a) 6 3 5 10 4
Br b) Br
CH2CH3 c) F
F AROMATIC ANSWERS page 2 AROMATIC ANSWERS
6
Cl a) Cl c) 1,2-dichlorobenzene Br CH3 p-bromomethylbenzene b) d)
Br Br Br 7 1,3,5-tribromobenzene OH
Br 3-bromo-5-chlorophenol Cl a) (trifluoromethyl)benzene d) 1-bromo-4-phenylheptaneo-, m-, and p- are used only w hen there are 2 substituents
b) 1,3-cyclohexadiene e) 1,2-diethyl-4-nitrobenzene
c) 1-phenyl-1-buten-3-yne f) 1-chloro-4-iodo-5-isopropyl-2-nitrobenzene
AROMATIC ANSWERS page 3 AROMATIC ANSWERS
2 Note that in all aromatic resonance structures, all C's are sp hybridized.
8 Limitations of F.C. alkylations include: a) Aromatic halides and vinyl halides will not react; only aliphatic halides (i.e., alkyl halides). b) Aromatics with substituents more deactivating (electron withdrawing) than a halogen inhibits the reaction. c) Polyalkylation occurs (producing mixed products) because the alkyl substituted product is more reactive than the aromatic starting material.
+ - - + d) The alkyl C intermediates may rearrange (by H: or CH3: shifts) to more stable C ’s. 9 Advantages of F.C. acylations over F.C. alkylations include: a) Aromatic acyl chlorides will react (along with aliphatic acyl halides). b) Polyalkylation does not occur. Only one major product results because the ketone product is less reactive than the aromatic starting material. c) No C+ rearrangements occur. 10 CH2Cl No, vinyl halides do not a) c) CH2 CHCl Yes, benzylic halides undergo F.C. alkylations. undergo F.C. alkylations. b) d)
Br 11 Yes, allylic halides CH2 CH CH2Cl No, aryl halides do not undergo F.C. alkylations. a) + e) CH3 wundergo ill not rearrange F.C. alkylations. CH3 1º rearranges 3º + b) f) + CH3CCH2 CH3CCH2CH3 methide CH CH3 shift c) + g) 3 CH3 CH3CH2 w ill not rearrange H neopentyl C+ 3º t-pentyl C+ rearranges H3C C + w ill not rearrange + CH CH d) + h) 3 H 3 CH3CHCH2 CH3CHCH3 1º rearrangest-butyl C+ hydride CH3 1º ++ CH CCH + shift 2º CH3CCH2 2º w ill not rearrange3 3 12 + w ill not rearrange CH(CH ) hydride + Cn-propylH3CHCCH CH32CH2CH3 isopropyl C+ 3 2 3º + H HCshiftl 2º isopropylbenzene + propylbenzene t-butyl C+ (cumene) isobutyl C+ minor product major product
13 + - a) H H2PO4 + Recall that the more highly substituted CH3CH CH CH3CH CH3 2 (more stable) Markovnikov C+ is formed. isopropyl C+
CH CH 3 + - 3 t-butyl C+ CH3 .. H HSO4 + H3C C OH H3C C OH2 H C C + AROMATIC ANSWERS ...... 3 page 4 - H2O.. CH3 CH3 CH3 AROMATIC ANSWERS
b)
O 14 O a) C AlCl3 + C Cl b) O c) O C AlCl3 CH3CH2CH2CH2O CH CH CH COH C Cl + 15 3 2 2 2 C AlCl 3 C NH NH2 a) d) Cl + 2 O + Br C Cl b) H e) Cl + O2N NH SO -2 m-bromobenzaldehyde o-chlorotoluene 3 4 p-chlorotoluene c) m-nitroanilinium sulfate 2
16 CH3 .. - CH3 .. - .. - :O : + :O: :O: :O: + C C C C SO3H HO3S CH3 CH3 CH3 CH3 o-methylbenzenesulfonic acid p-methylbenzenesulfonic+ acid+
acetophenone o- and p- positions are electron deficient. This deactivating group is m-directing
+ 17 .. - + NH2 + .. NH2 NH2 NH2 :- - : o- and p- positions are electron rich. This activating group is o-,p-directing.
18 O F NH + Br a) C NH2 3 H 2 1 5 3 4 b) O c) OH Cl CH2CH3 C OH O 3 2 1 O 19 F.C. acylations add a 4ring deactivating carbonyl group to the aromatic ring. The product is thus less reactive than NO I the aromatic reagent.C The product will not react under2 normal conditions. NH C CH3 CH 3 3 2 1 O 4 NH C CH3 AROMATIC ANSWERS page 5
OH AROMATIC ANSWERS
F.C. alkylations add a ring activating alkyl group to the aromatic ring. The product is more reactive than the aromatic reagent. The product will react producing several substitution products. 20 H3CO NO2 a) c)
b) d) O COOH C CH3
F
AROMATIC ANSWERS page 6 AROMATIC ANSWERS
O O N(CH3)2 NH2 NH C CH3 O C CH3
increasing reactivity toward EAS
N is less electronegative than O, so N bonded to the aromatic ring is a better electron donor than O. Hence acetanilide is more reactive than phenyl acetate. In acetanilide, the NH is bonded to an electron withdrawing carbonyl group and is less able to donate electrons to the ring than in aniline. Alkyl groups are electron donors. Thus N,N-dimethylaniline is more electron donating than is aniline. 21 O O a) O C KMnO4 C CH2CH3 COOH + H2O, H C OH
CH3 COOH
O b) KMnO (CH3CH2)3 C CH(CH2CH3)2 4 (CH3CH2)3 C COOH + 2 CH3CH2 C OH H2O,
22
Cl KOH in ethanol AlCl3
Br H NBS
(PhCOO)2
23 F.C. alkylations involving 1º C+’s such as isobutyl will inevitably rearrange producing some 3º C+. However, F.C.
acylation produces only one product. The carbonyl product can subsequently be reduced over H2
CH3
CH2CHCH3
AlCl3 O CH3 CH3 O C H2/Pd CHCH3 CH3CHC Cl
AROMATIC ANSWERS page 7 AROMATIC ANSWERS
24
Cl , AlCl3 2 I2 HNO3 H2SO4 AlCl3 FeCl CH3COCl 3 HNO3 H2SO4 SO3 CH3CH2Cl O Cl I NO2 SO3H CH2CH3 C CH3
H2/Pt H2/Pt 1. NaOH, 2. H2O NBS (PhCOO) OH NH 2 2 OH CHBrCH3 CHCH3
OH HOCl KOH H2SO4 CH CH2Cl HBr ethanol H2O CH2CH2OH CH=CH2 CHBrCH2Br 1. BH3, THF, H2O 2. - Br2 H2O2, OH HBr NaNH2 KMnO4, H2O2 NH3 , H2O CH CH Br cold 2 2 C CH KMnO4, COOH 2HCl H2O Cl HgSO4, O OH + H O+ C 3 HCOOH CH3 C CHCH2OH CH3 Cl
25 CH3 CH3 CH3 CH3CH2Cl CH CH a) + 3 2 AlCl Cl Cl 3 Cl CH2CH3 1-chloro-2-ethyl-5-methylbenzene 1-ethyl-4-chloro-2-methylbenzene
b) O NH2 CH3CCl no reaction, amino group reacts with AlCl3
AlCl3
c) COOH CH3Cl no reaction, ring too deactivated
AlCl3
AROMATIC ANSWERS page 8 AROMATIC ANSWERS
26 O a) C H2/Pd CH CH CH CH2CH3 2 2 3
O2N H N CH CH2 2 CH2CH3
O b) N2H4 C KOH CH2CH2CH3 CH2CH3
O2N CH H N CH2 2 CH CH2
27 CH a) 3 Note: alkylation must precede formation of -NH2 group because -NH2 reacts w ith AlCl 3 O H /Pd AlCl3 NH2 2 HC Cl
O HNO3 CH3 H2/Pd CH3 C H H2SO4 O2N
COOH
b) Alkylation must precede sulfonation for two reasons. 1. The -SO3H group is meta directing SO3H AlCl3 2. The -SO3H group would inhibit alkylation
CH3Cl KMnO4, H2O
CH3 H2SO4 CH3 SO3
SO3H
OH c)
SO3H H2SO4 SO3 H2SO4 The -OH group is o-, p-directing. SO3 SO H 3 OH
1. 300ºC, NaOH 2. H3O+
AROMATIC ANSWERS page 9 AROMATIC ANSWERS
d) CH3
AlCl3 The methyl group must not be AlCl H3C C CH2 3 CH3Cl present when bromination CH(CH3)2Cl is conducted otherwise it will also be brominated. NBS KOH (PhCOO)2 ethanol
H C CH H3C C CH2 3 CH3 H3C CBr CH3
CH CH CH CH e) 2 2 2 3
The acyl group should be reduced Br only after brominating since a O AlCl3 m-directing group is needed to N2H4 cause bromination in the meta Cl CCH2CH2CH3 KOH position. The acyl group is later reduced to an alkyl group. In addition, an n-butyl group would O O rearrange if F.C. alkylation were attempted directly. CCH2CH2CH3 CCH2CH2CH3 Br2 FeBr3
Br
SO3H f) CH2CH(CH3)2
H2SO4 SO3 O AlCl3 Br Cl CCH(CH ) 3 2 O O Br2 Zn[Hg] CH2CH(CH3)2 CCH(CH3)2 HCl CCH(CH3)2 FeBr3 Br Br
g) KOH Br ethanol NO NO2 Br 2
CH CH2 CHBrCH3 CH3CH2Cl
HNO3 AlCl3 H2SO4 Br2 NBS Br FeBr3 (PhCOO)2 CH2CH3 CHBrCH3 CHBrCH3
AROMATIC ANSWERS page 10 AROMATIC ANSWERS
28
:O: : O : :O: ...... R C N : R NH R C Cl : R C H 2 R C .O. H ..
nitrile 1º amine carboxylic acid acid chloride aldehyde (acyl chloride)
.. : O : .. + O .. .. R N .. OH NH C CH3 .. - R .O. H .. O : .. 2º amide nitro compound alcohol phenol
Recall that Group 5A elements (N, P, etc.) normally have 1 lone pair. Group 6A elements (O, S, etc.) normally have 2 lone pairs. Group 7A elements (F, Cl, Br, I) normally have 3 lone pairs of electrons. Group 4A elements (C, Si, etc.) normally have no lone pairs of electrons.
29
FeBr3 H H + H Br Br Br + Br Br + slow + + double allylic C+
carbocation intermediate (arenium ion or -complex)
H Br Br - FeBr + FeBr4 + HBr + 3 + fast
AROMATIC ANSWERS page 11