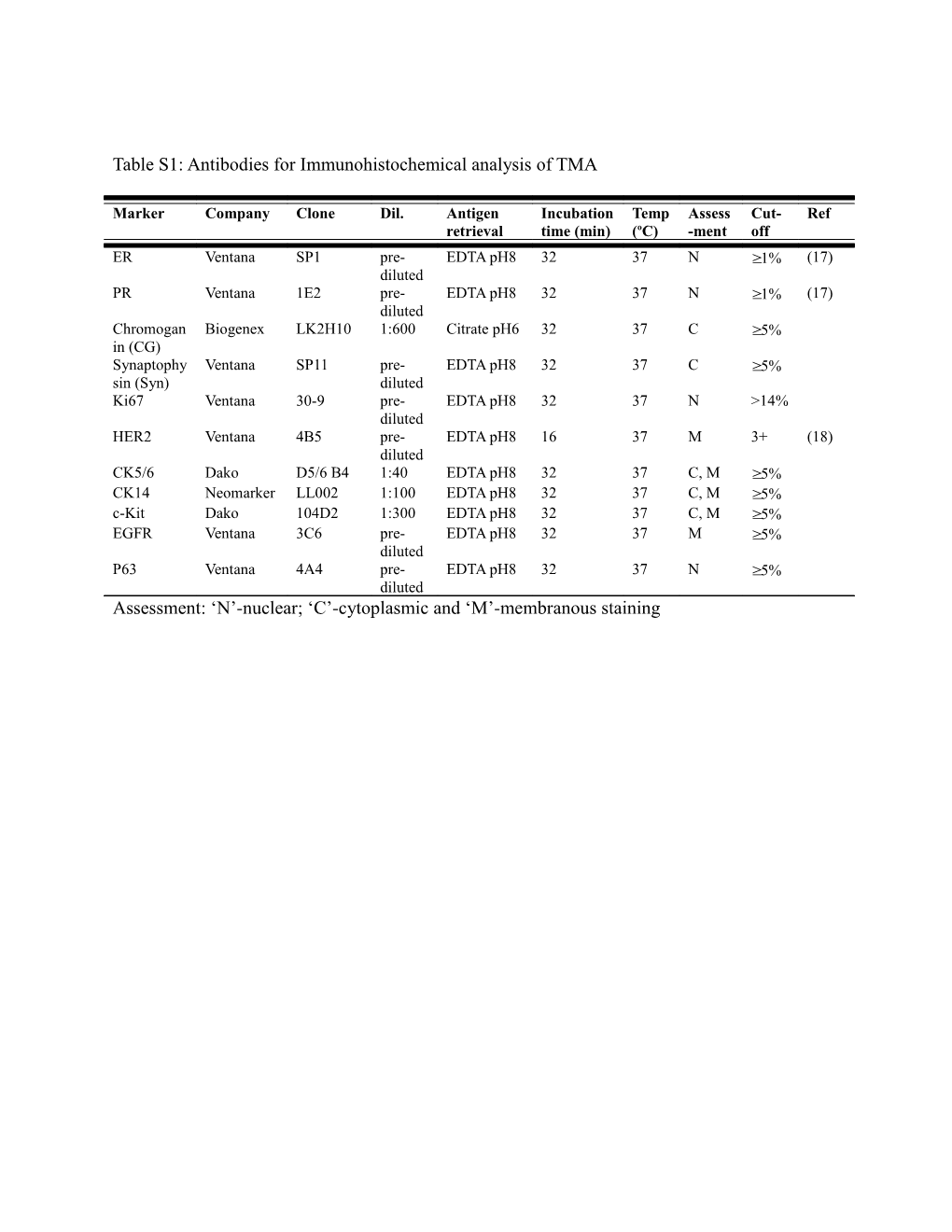
Table S1: Antibodies for Immunohistochemical analysis of TMA
Marker Company Clone Dil. Antigen Incubation Temp Assess Cut- Ref retrieval time (min) (oC) -ment off ER Ventana SP1 pre- EDTA pH8 32 37 N ≥1% (17) diluted PR Ventana 1E2 pre- EDTA pH8 32 37 N ≥1% (17) diluted Chromogan Biogenex LK2H10 1:600 Citrate pH6 32 37 C ≥5% in (CG) Synaptophy Ventana SP11 pre- EDTA pH8 32 37 C ≥5% sin (Syn) diluted Ki67 Ventana 30-9 pre- EDTA pH8 32 37 N >14% diluted HER2 Ventana 4B5 pre- EDTA pH8 16 37 M 3+ (18) diluted CK5/6 Dako D5/6 B4 1:40 EDTA pH8 32 37 C, M ≥5% CK14 Neomarker LL002 1:100 EDTA pH8 32 37 C, M ≥5% c-Kit Dako 104D2 1:300 EDTA pH8 32 37 C, M ≥5% EGFR Ventana 3C6 pre- EDTA pH8 32 37 M ≥5% diluted P63 Ventana 4A4 pre- EDTA pH8 32 37 N ≥5% diluted Assessment: ‘N’-nuclear; ‘C’-cytoplasmic and ‘M’-membranous staining Table S2 Multivariate cox regression analysis of clinic-pathological parameters and the presence of fibrotic focus on disease free survival Clinico-pathological Hazard ratio 95% confident interval p-value parameters LVI 2.239 1.086-4.612 0.029 Lymph node involvement 4.426 1.835-10.680 0.001 FF 2.570 1.267-5.214 0.009 Grade 3.024 1.717-5.323 <0.001 Tumor size, age, EIC, ER and HER2 status were not statistically significant predictors of disease free survival by backward ward multivariate analysis.