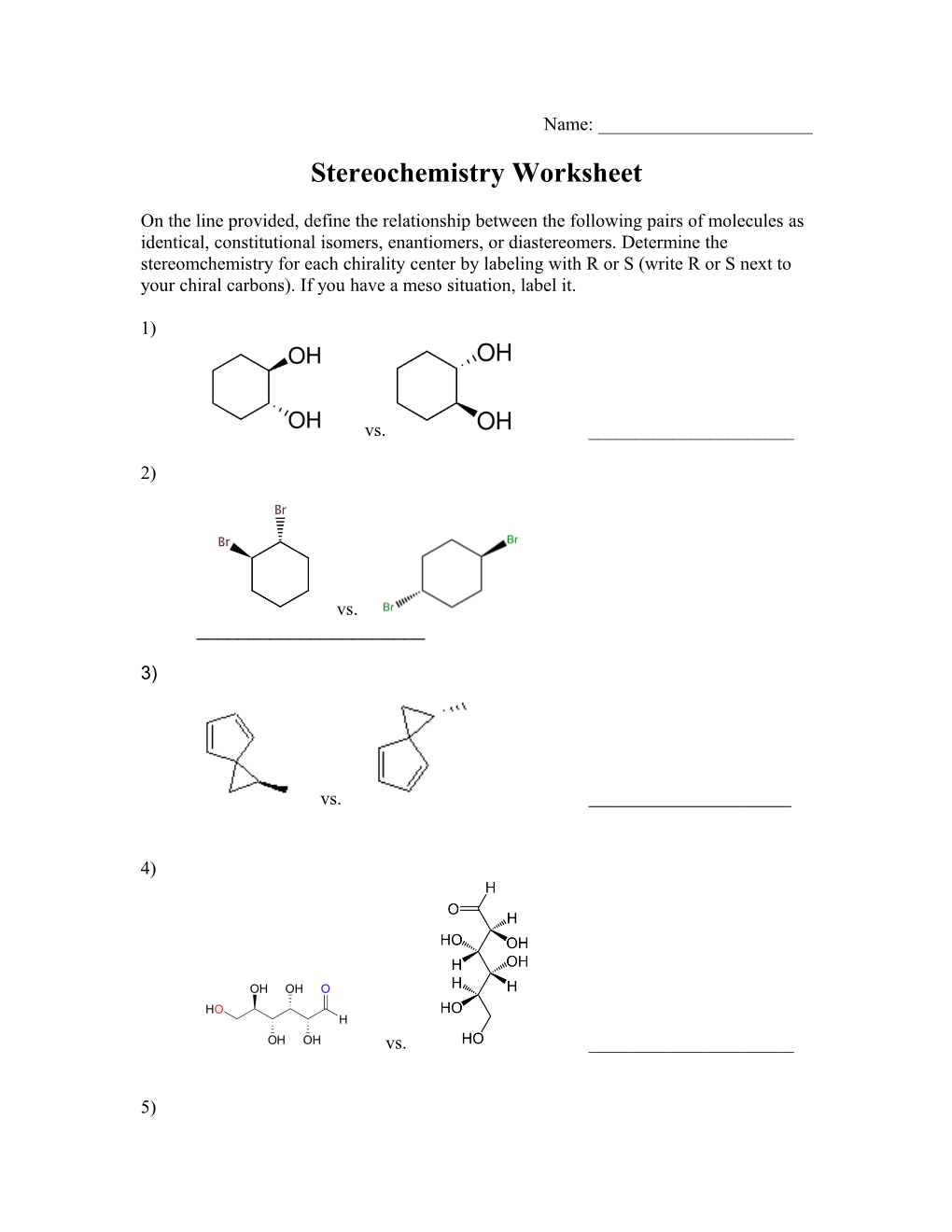
Name: ______Stereochemistry Worksheet
On the line provided, define the relationship between the following pairs of molecules as identical, constitutional isomers, enantiomers, or diastereomers. Determine the stereomchemistry for each chirality center by labeling with R or S (write R or S next to your chiral carbons). If you have a meso situation, label it.
1)
vs. ______
2)
vs. ______
3)
vs. ______
4)
vs. ______
5) vs. ______
6) Ibuprofen is the active ingredient in Motrin, Nuprin, and Advil. It is currently sold as a racemic mixture although the S enantiomer is the active pain reliever and the R enantiomer is inactive. Draw 3D pictures of these two enantiomers. Label the chiral carbon and indicate which is the R and which is the S
7) Explain why there are different physiological effects with the different enantiomers of ibuprofen.
8) Can you tell, just by inspecting the ibuprofen enantiomers, which one will be dextrorotatory and which will be levorotatory? How might you distinguish between the two isomers of ibuprofen in the lab?
9) Draw all the stereoisomers of 2,3-dibromobutane. Which are enantiomers? Which are diastereomers? Are any identical/meso?
10) Classify the following as “optically active” or “optically inactive.” a) A solution of 1-iodohexane b) A solution of (S)-2-iodohexane c) A solution of 40% (S)-3-chlorohexane and 60% (R)-3-chlorohexane d) A solution of equal quantities of R and S 3-chlorohexane