Matter and Measurements
Types of Matter
Matter is anything that has mass and occupies space.
Matter exists in three phases:
(1) solid- fixed shape and volume (2) liquid – fixed volume but not rigid in shape (3) gas – neither a fixed volume nor rigid shape
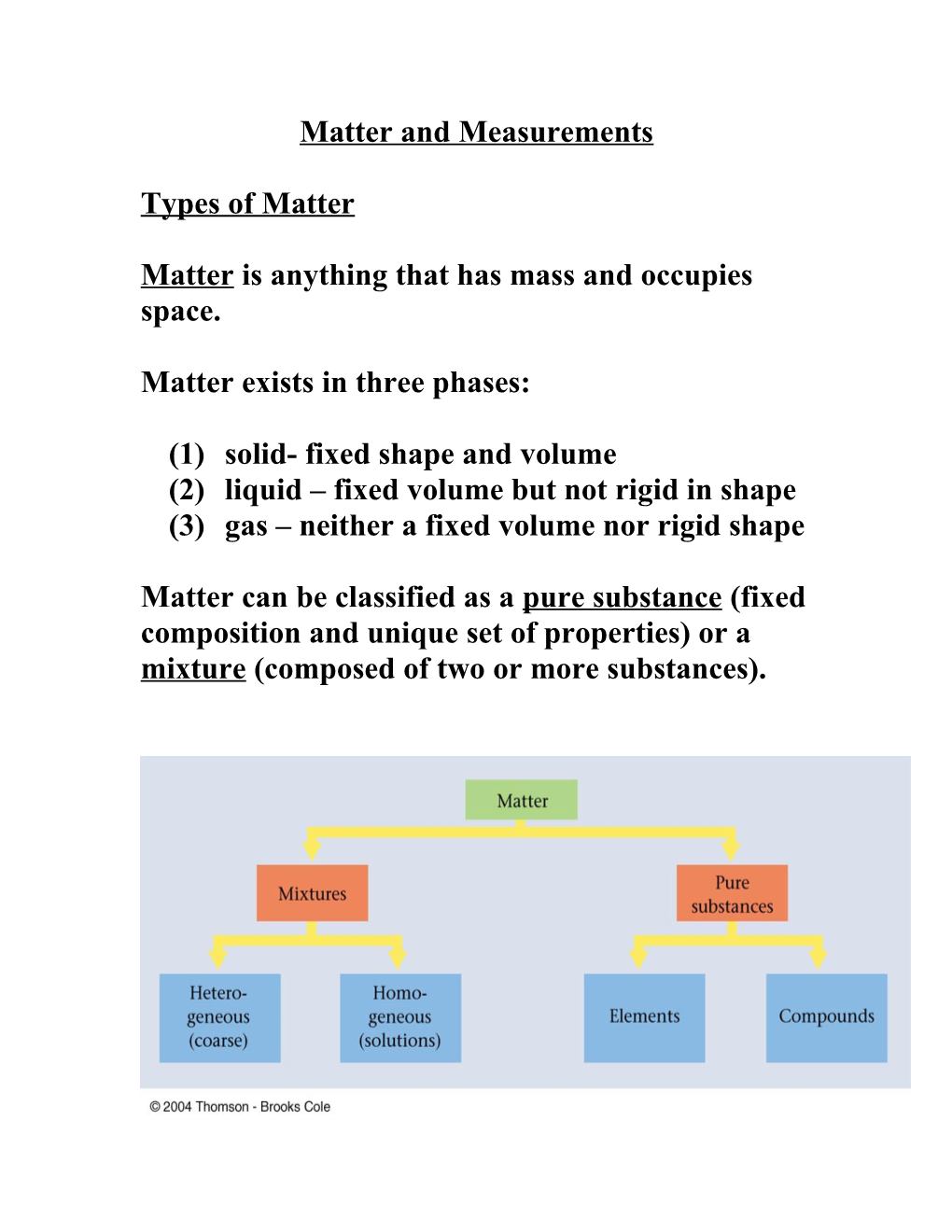
Matter can be classified as a pure substance (fixed composition and unique set of properties) or a mixture (composed of two or more substances). An element is a type of matter that cannot be broken down into two or more pure substances.
There are ______known elements. 91 of those elements occur naturally.
Each element can be identified by its symbol, which consists of one or two letters, usually derived from the name of the element.
Example: Al is aluminum
The symbol may also be from the Latin name of the element or one of its compounds.
Example: Cu is from Cuprum
You must learn the symbols for the common elements.
A compound is a pure substance that contains more than one element.
Example: CH4 – methane
Compounds have fixed compositions. A compound always contains the same elements in the same percentages by mass. Example: Pure water is 11.19 % H and 88.81 % oxygen by mass.
The properties of compounds are very different from those of the elements they contain.
Many different methods (heat, light, and electrolysis) can be used to resolve compounds into their elements.
A mixture contains two or more substances combined in such a way that each substance retains its chemical identity.
There are two types of mixtures:
(1) Homogeneous - (uniform) the composition is the same throughout.
Examples – sodium chloride solution, brass
(2) Heterogeneous - (nonuniform) the composition varies throughout.
Example: granite (rock containing the minerals feldspar, mica, and quartz) There are many ways to separate the components of a mixture such as filtration (heterogeneous s-l mixtures) and distillation (homogeneous s-l mixture).
Another method is chromatography, which takes advantage of differences in solubility and/or the extent of adsorption on a solid surface. In gas-liquid chromatography, a mixture of volatile liquids and gases is introduced into one end of a heated glass tube. The tube is packed with an inert solid whose surface is coated with a viscous liquid. An unreactive carrier gas (He) is passed through the tube. The components of the sample gradually separate as they vaporize into the carrier or condense into the viscous liquid.
Usually more volatile fractions move faster and emerge first. The following plot for natural gas is typical. The area under each peak is determined and translated into a concentration. Measurements
Chemistry is a quantitative science. Scientific measurements are expressed in the metric system, the decimal-based system in which all of the units of a particular quantity are related to one another by factors of 10. Common Metric Prefixes yotta- (Y-) 1024 1 septillion zetta- (Z-) 1021 1 sextillion exa- (E-) 1018 1 quintillion peta- (P-) 1015 1 quadrillion tera- (T-) 1012 1 trillion giga- (G-) 109 1 billion mega- (M-) 106 1 million kilo- (k-) 103 1 thousand hecto- (h-) 102 1 hundred deka- (da-) 10 1 ten deci- (d-) 10-1 1 tenth centi- (c-) 10-2 1 hundredth milli- (m-) 10-3 1 thousandth micro- (µ-) 10-6 1 millionth nano- (n-) 10-9 1 billionth pico- (p-) 10-12 1 trillionth femto- (f-) 10-15 1 quadrillionth atto- (a-) 10-18 1 quintillionth zepto- (z-) 10-21 1 sextillionth yocto- (y-) 10-24 1 septillionth The standard unit of length in the metric system is the meter (m).
A meter is defined as the distance light travels in 1/299,792,458 of a second.
Volume is commonly expressed in cubic centimeters (cm3), liters (L), and milliliters (mL).
1 mL = 1 cm3 1 L = 1 dm3 1 L = 1.057 qt 1 gal = 4 qts = 8 pt
Mass is most commonly expressed in grams (g), kilograms (kg), or milligrams (mg).
1 lb = 453.6 g = 16 oz 1 kg = 2.2 lbs.
There is a distinction between mass and weight.
Mass is a measure of the amount of matter in an object.
Weight (SI unit is N) is a measure of the gravitational force acting on an object. W= mg
Temperature is the factor that determines the direction of heat flow. It is a measure of the average kinetic energy in the particles of a sample.
Heat always flows from an object of higher temperature to one having a lower temperature.
Temperature is commonly measured in degrees Celsius (centigrade) or in the US Fahrenheit degrees.
0 t0F = 1.8 t0C + 32 0 t0C = 0.56 (t0F -32 )
Kelvin (K) is defined to be 1/273.16 of the difference between the lowest obtainable temperature (O K) and the triple point of water (0.010C). tk = t0C + 273.15 Example Mercury thermometers are being phased out because of the toxicity of mercury vapor. A common replacement for mercury is isoamyl benzoate, which boils at 2620C. What is its boiling point in 0F and K?
The SI units are:
Name Symbol Quantity
meter m length kilogram kg mass second s time ampere A electric current
kelvin K thermodynamic temperature
mole mol amount of substance candela cd luminous intensity Every measurement carries a degree of uncertainty whose magnitude depends on the nature of the measuring device and the skill with which it is used.
Example: If a graduated cylinder is uncertain to +/- 1 mL an 8 mL reading would be between 7 and 9 mL.
8 +/- 1mL or 8 mL = large graduated cylinder
8.0 +/- 0.1 mL or 8.0 mL = small graduated cylinder
8.00 +/- 0.01 mL or 8.00 mL = buret
When the +/- notation is dropped, it is understood that there is an uncertainty of at least one unit in the last digit.
This method of citing the degree of confidence in a measurement is described in terms of significant figures, the meaningful digits obtained in a measurement. Precision refers to how closely two or more measurements of the same quantity agree with one another.
Accuracy tells us how close a measurement is to the true value of the quantity measured.
Accuracy without Precision
Precision without Accuracy
Rules for Determining sig. figs: (1) Read the number from left to right starting with the first non-zero digit.
215.6 = 4 sig. figs. 2015.6 = 5 sig. figs. (2) Zeros holding the decimal place are not significant.
300 = 1 sig. fig. 0.005 = 1 sig. fig. 300. = 3 sig. figs. 306 = 3 sig. figs. 0.0050 = 2 sig. figs. 300.1 = 4 sig. figs.
(3) The number of significant figures equals the number of digits shown when exponential notation (scientific notation) is used.
5.00 x 102 = 3 sig. figs. 5 x 102 = 1 sig fig.
(4) When measured quantities are multiplied or divided, the number of significant figures in the answer should be that same as the quantity with the smallest number of sig. figs. 2.4 x 1 = 2.4 = 2
(5) When adding or subtracting, the number of decimal places in the result is the same as the quantity with the greatest uncertainty (smallest # of decimal places). 2.5 + 1 = 3.5 = 4 (6) Numbers spelled out indicate an exact amount. One gram of coal was burned.
Rules for rounding: (a) If the digits to be discarded are less than - - 500…, leave the last digit unchanged. 23.315 and 23.487 rounded to 2 sig. figs. = 23 (b) If the digits to be discarded are greater than - - 500…, add one to the last digit. 23.692 and 23.514 rounded to 2 sig. figs. = 24 (c) If the digits to be discarded is exactly - - 500…, round off the last digit to an even number. 23.500 and 24.5 rounded to 2 sig. figs. = 24 Example
Using different balances, three different students weigh the same object. They report the following masses: (a) 1.611 g (b) 1.60 g (c) 0.001611 kg How many sig. figs. does each value have?
Example A flight leaves Philadelphia and arrives in Frankfurt 8.05 hours later. The airplane travels a distance of 6.6 x 103 km. What is the average speed in kilometers per hour?
Dimensional analysis or the factor label method is used to convert from a given to a desired unit.
In general, when you make a conversion, chose the factor that cancels out the initial unit. Example A filling station in Paris sells gas for 1.18 euros per liter. Assume one dollar = 1.15 euro. (a) How much will a full tank of gas (43L) cost? (b) Suppose you have 9L of gas in your tank and fill up, how much would you pay in dollars? (c) What is the cost of the gas in dollars per gallon?
Properties of Substances
Properties used to identify a substance are intensive (independent of amount). Example: color
Properties that tell us nothing about a substance’s identity (and depend on amount) are extensive.
Example: mass, volume
Substances may also be identified by:
(1) Chemical properties, observed when a substance takes part in a chemical reaction (a change that converts it to a new substance).
Examples: chemical inertness of He, reaction of sodium hydroxide and hydrochloric acid (neutralization)
(2) Physical properties, observed without changing the chemical identity of a substance.
Examples: melting point, taste, boiling point, color, density, solubility, absorption spectrum
Density is the ratio of mass to volume.
Density = mass / volume
d or ρ = m/v Example To determine whether a cylindrical “copper” bar is pure copper, an assayer weighs it, finding a mass of 123 g. He then measures the length of the bar to be 12.0 cm and its diameter to be 1.24 cm. What is the density of the bar?
Solubility is the process by which a solute dissolves in a solvent.
When temperature changes, the amount of solute in solution changes, but the mass of the H2O stays the same.
Example Taking the solubility of sugar (sucrose) to be 487 g/100 g of water at 100 0 C and 204 g/100 g water at 20 0C, calculate: (a) the mass of water required to dissolve one hundred grams of sugar at 100 0C. (b) the amount of sugar that remains in solution when the mixture in (a) is cooled to 20 0C. This figure shows the solubility of sugar in water as a function of temperature. It gives the concentration of sugar in a saturated solution at various temperatures. At any point in the area below the curve, we are dealing with an unsaturated solution. At any point in the area above the curve, the sugar solution is supersaturated.
Color - Some substance can be indentified (tentatively) by their color.
The colors of gases and liquids are due to the selective absorption of certain components of visible light (400 to 700 nm).
The subtraction of the absorbed colors from visible light account for the color we see.
Colorless substances absorb in the UV( ‹ 400 nm) or IR ( › 700 nm) region. Example: KMnO4 absorption in the green region produces a blue-red color.