The cooling curve of cetyl alcohol
AIM The aim of this experiment is to investigate the cooling of a liquid and to find the temperature at which it solidifies.
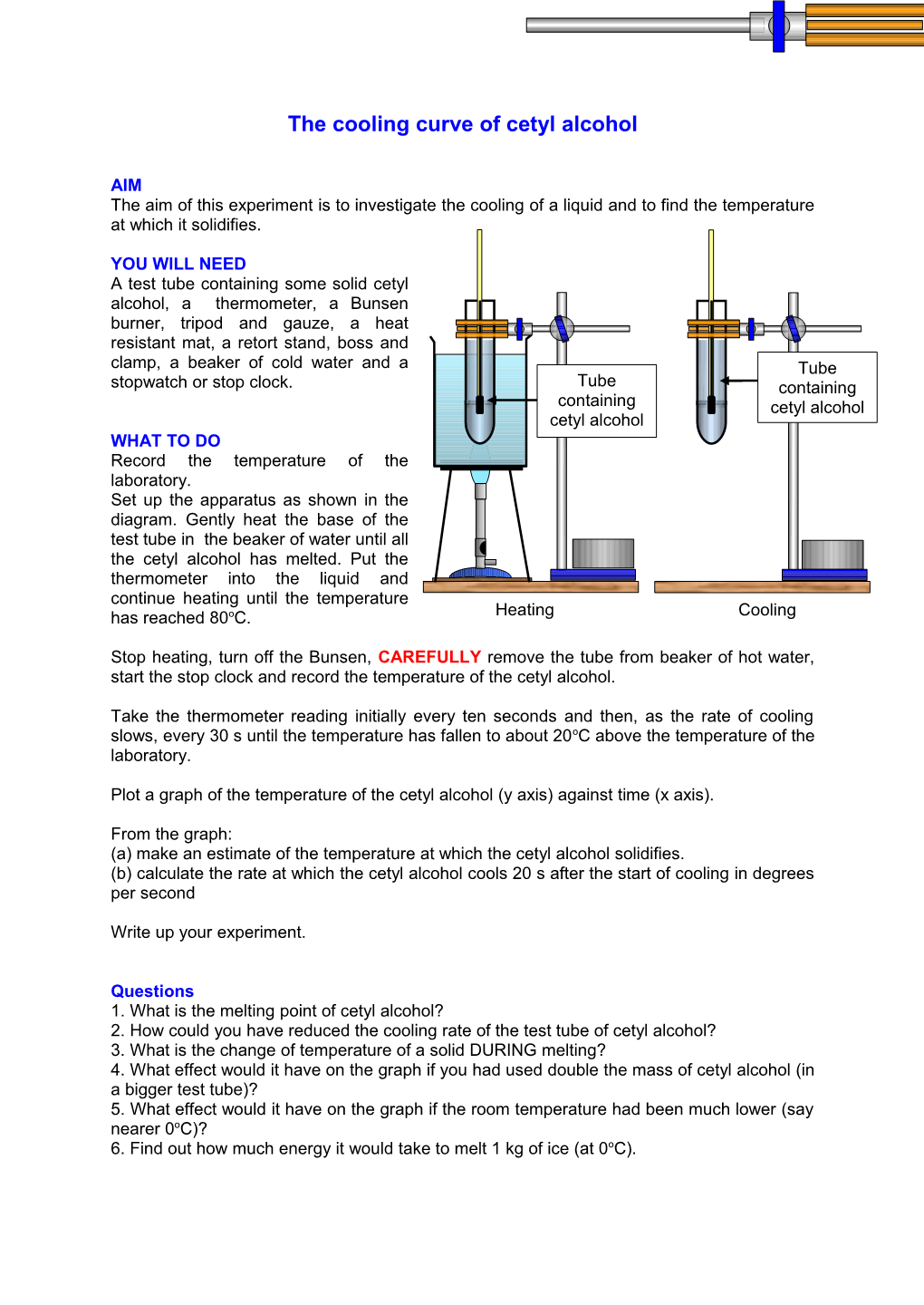
YOU WILL NEED A test tube containing some solid cetyl alcohol, a thermometer, a Bunsen burner, tripod and gauze, a heat resistant mat, a retort stand, boss and clamp, a beaker of cold water and a Tube stopwatch or stop clock. Tube containing containing cetyl alcohol cetyl alcohol WHAT TO DO Record the temperature of the laboratory. Set up the apparatus as shown in the diagram. Gently heat the base of the test tube in the beaker of water until all the cetyl alcohol has melted. Put the thermometer into the liquid and continue heating until the temperature has reached 80oC. Heating Cooling
Stop heating, turn off the Bunsen, CAREFULLY remove the tube from beaker of hot water, start the stop clock and record the temperature of the cetyl alcohol.
Take the thermometer reading initially every ten seconds and then, as the rate of cooling slows, every 30 s until the temperature has fallen to about 20oC above the temperature of the laboratory.
Plot a graph of the temperature of the cetyl alcohol (y axis) against time (x axis).
From the graph: (a) make an estimate of the temperature at which the cetyl alcohol solidifies. (b) calculate the rate at which the cetyl alcohol cools 20 s after the start of cooling in degrees per second
Write up your experiment.
Questions 1. What is the melting point of cetyl alcohol? 2. How could you have reduced the cooling rate of the test tube of cetyl alcohol? 3. What is the change of temperature of a solid DURING melting? 4. What effect would it have on the graph if you had used double the mass of cetyl alcohol (in a bigger test tube)? 5. What effect would it have on the graph if the room temperature had been much lower (say nearer 0oC)? 6. Find out how much energy it would take to melt 1 kg of ice (at 0oC).