CHEM 109A- Zhang CLAS W10 Acid Strength “Effects”/Trends for predicting pKas & relative Acid/Base Strength You can use the following effects to explain/compare acid (and base) strengths Element Effects (depend on the atom that the acidic H is bound to) EN (electronegativity) – use when elements are related horizontally/in same period As EN of element increases the acid strength increases (more polar bond is weaker and H is more likely to be donated/pulled off). HF > H2O > NH3 EN of atom in bold 4.0 3.5 3.0 from T10.3 in text
EN = ENatom-ENH 1.9 1.4 0.9 measure of bond polarity (larger diff = more polar) pKa of acid 3.2 15.7 36 smaller pKa = stronger acid
Size/BDE (bond dissociation energy) – use when elements are related vertically/in same family As BDE (energy required to break the bond) decreases the acid strength increases (the weaker the bond, the easier it is to donate/pulled off a H atom). Can think of this as a size effect b/c know the periodic trend for size & larger the atom, the longer and weaker the bond. HI > HBr > HCl > HF Atomic radius atom in bold 133 114 99 64 pm from F12.38 Zumdahl Approx bond length 170 151 136 101 pm, atomic radius H = 37 pm Approx BDE 71 87 103 135 kcal/mol pKa -10 -9 -7 3.2
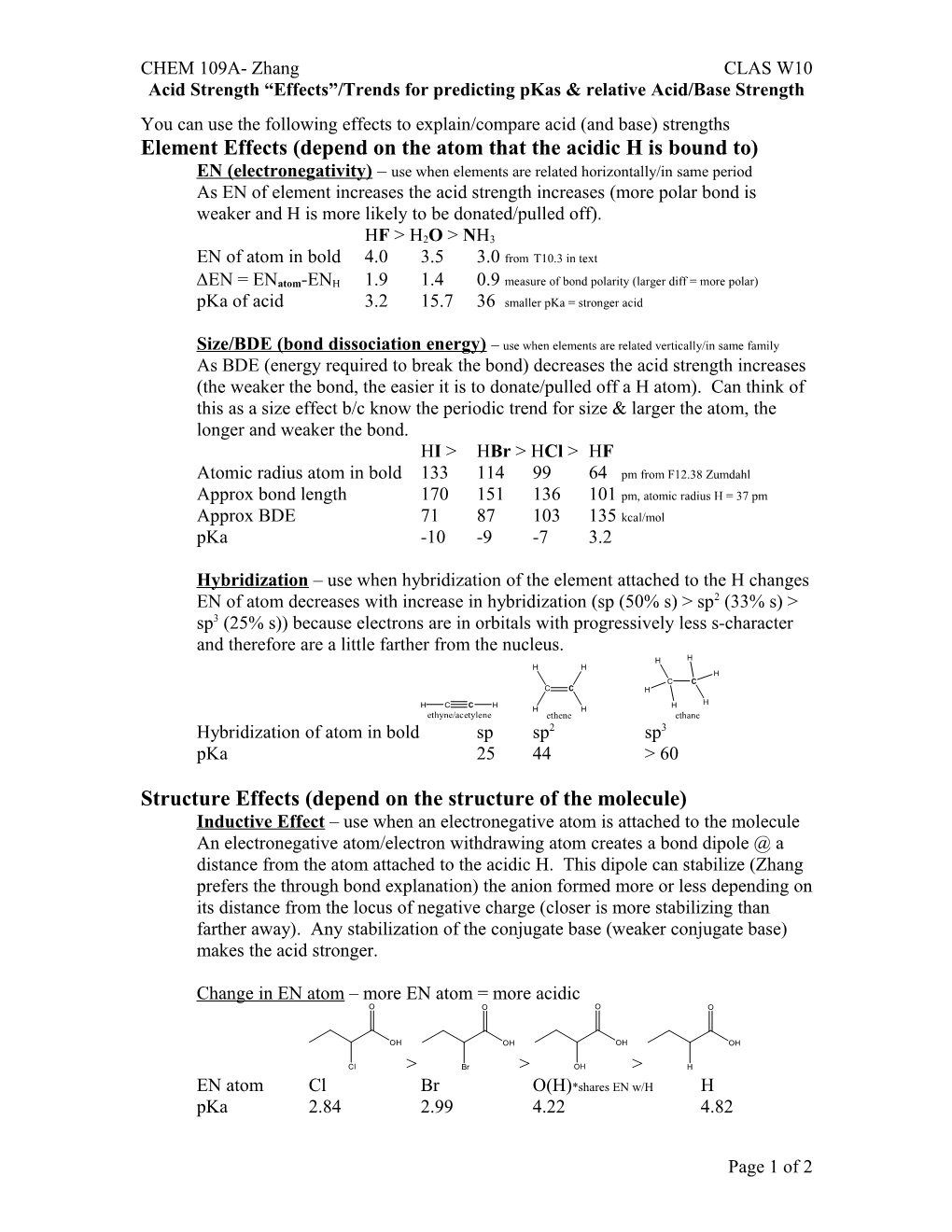
Hybridization – use when hybridization of the element attached to the H changes EN of atom decreases with increase in hybridization (sp (50% s) > sp2 (33% s) > sp3 (25% s)) because electrons are in orbitals with progressively less s-character and therefore are a little farther from the nucleus. H H H H H C C C C H
H C C H H H H H ethyne/acetylene ethene ethane Hybridization of atom in bold sp sp2 sp3 pKa 25 44 > 60
Structure Effects (depend on the structure of the molecule) Inductive Effect – use when an electronegative atom is attached to the molecule An electronegative atom/electron withdrawing atom creates a bond dipole @ a distance from the atom attached to the acidic H. This dipole can stabilize (Zhang prefers the through bond explanation) the anion formed more or less depending on its distance from the locus of negative charge (closer is more stabilizing than farther away). Any stabilization of the conjugate base (weaker conjugate base) makes the acid stronger.
Change in EN atom – more EN atom = more acidic O O O O
OH OH OH OH
Cl > Br > OH > H EN atom Cl Br O(H)*shares EN w/H H pKa 2.84 2.99 4.22 4.82
Page 1 of 2 CHEM 109A- Zhang CLAS W10 Acid Strength “Effects”/Trends for predicting pKas & relative Acid/Base Strength Change in distance from acidic H – closer to acidic H = more acidic O
Cl O O O OH Cl Cl > OH > OH > OH pKa 2.86 4.05 4.53 4.82
Change in # of EN atom – more EN atoms = more acidic Cl Cl OH OH Cl Cl Cl > Cl > OH > OH # of EN atom 3 2 1 0 pKa 12.24 12.31 12.86 16.0
Resonance Effect – use when can drawn resonance structures for the conjugate base The more valid (obey octet rule) resonance structures you can draw for the conjugate base the more stable it is (less basic) and the stronger the acid. O
OH OH O
> OH > > OH pKa 4.2 4.8 10.0 16.0
What other effect might be present in the first two structures listed above?
Also know how to use these effects/trends to compare base strength Remember, the stronger the acid, the weaker the conjugate base
H H H EX. Circle the stronger base amide ion -N or hydroxide ion -O Might want to first answer: Which is the stronger acid?
It is a good idea to know the following pKa values, but Zhang will provide pKa values Hydronium ion -1.7 = -2 Acetic acid 4.8 = 5 Ammonium ion 9.4 = 9 Methanol (MeOH) 15.5 = 15 Water 15.7 = 16 Ammonia 36
Acid form (HA) or base form (A-) in solution? Depends on pH of solution and pKa of HA: pKa = pH - log([A-]/[HA])
[HA] or [A - ] = 10 pH-pKa pH < pKa more acidic than pKa HA form pH = pKa [HA] = [A-], 50/50 pH > pKa less acidic/more basic than pKa A- form
Page 2 of 2