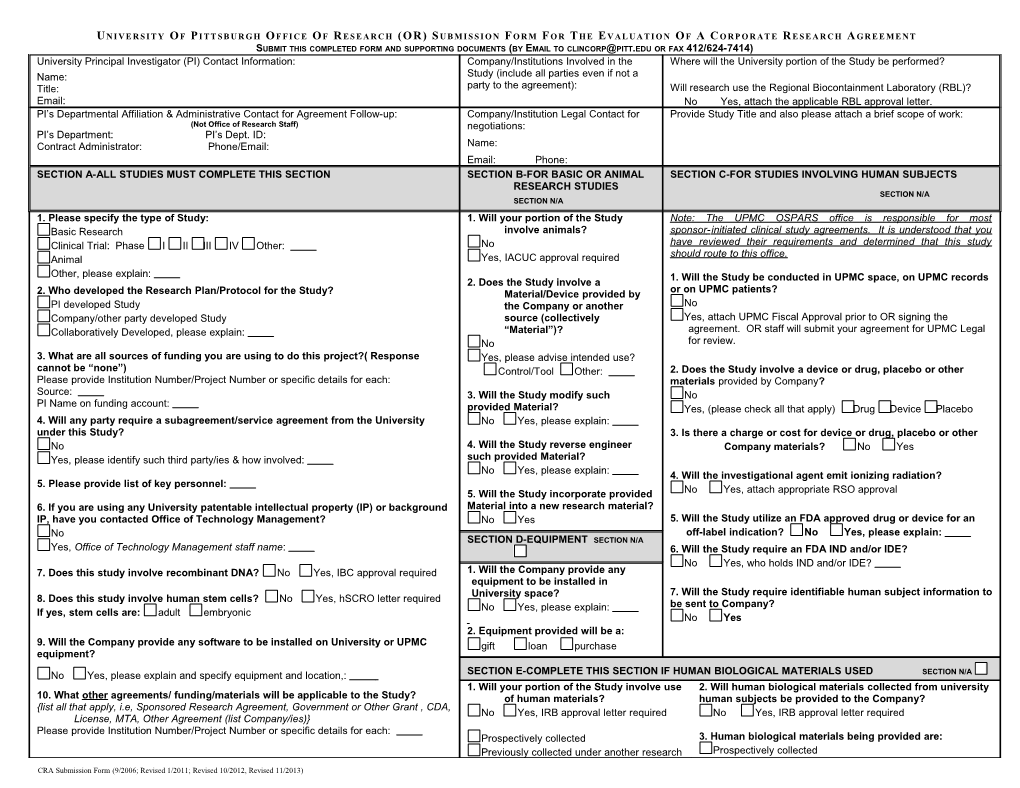
U N I VE R S I T Y O F P I T T S B U R G H O F F I C E O F R E S E A R C H (OR) S U B M I S S I O N F O R M F O R T H E E V AL U A T I O N O F A C O RP O R AT E R E S E A R C H A G R E E M E NT SUBMIT THIS COMPLETED FORM AND SUPPORTING DOCUMENTS (BY EMAIL TO [email protected] OR FAX 412/624-7414) University Principal Investigator (PI) Contact Information: Company/Institutions Involved in the Where will the University portion of the Study be performed? Name: Study (include all parties even if not a Title: party to the agreement): Will research use the Regional Biocontainment Laboratory (RBL)?
Email: No Yes, attach the applicable RBL approval letter. PI’s Departmental Affiliation & Administrative Contact for Agreement Follow-up: Company/Institution Legal Contact for Provide Study Title and also please attach a brief scope of work: (Not Office of Research Staff) negotiations: PI’s Department: PI’s Dept. ID: Contract Administrator: Phone/Email: Name: Email: Phone: SECTION A-ALL STUDIES MUST COMPLETE THIS SECTION SECTION B-FOR BASIC OR ANIMAL SECTION C-FOR STUDIES INVOLVING HUMAN SUBJECTS RESEARCH STUDIES SECTION N/A SECTION N/A 1. Please specify the type of Study: 1. Will your portion of the Study Note: The UPMC OSPARS office is responsible for most Basic Research involve animals? sponsor - initiated clinical study agreements. It is understood that you Clinical Trial: Phase I II III IV Other: No have reviewed their requirements and determined that this study should route to this office. Animal Yes, IACUC approval required Other, please explain: 2. Does the Study involve a 1. Will the Study be conducted in UPMC space, on UPMC records 2. Who developed the Research Plan/Protocol for the Study? Material/Device provided by or on UPMC patients? PI developed Study the Company or another No Company/other party developed Study source (collectively Yes, attach UPMC Fiscal Approval prior to OR signing the Collaboratively Developed, please explain: “Material”)? agreement. OR staff will submit your agreement for UPMC Legal No for review. 3. What are all sources of funding you are using to do this project?( Response Yes, please advise intended use? cannot be “none”) Control/Tool Other: 2. Does the Study involve a device or drug, placebo or other Please provide Institution Number/Project Number or specific details for each: materials provided by Company? Source: 3. Will the Study modify such No PI Name on funding account: provided Material? Yes, (please check all that apply) Drug Device Placebo 4. Will any party require a subagreement/service agreement from the University No Yes, please explain: under this Study? 3. Is there a charge or cost for device or drug, placebo or other No 4. Will the Study reverse engineer Company materials? No Yes Yes, please identify such third party/ies & how involved: such provided Material? No Yes, please explain: 4. Will the investigational agent emit ionizing radiation? 5. Please provide list of key personnel: 5. Will the Study incorporate provided No Yes, attach appropriate RSO approval 6. If you are using any University patentable intellectual property (IP) or background Material into a new research material? IP, have you contacted Office of Technology Management? No Yes 5. Will the Study utilize an FDA approved drug or device for an No off-label indication? No Yes, please explain: SECTION D-EQUIPMENT SECTION N/A Yes, Office of Technology Management staff name: 6. Will the Study require an FDA IND and/or IDE? No Yes, who holds IND and/or IDE? 7. Does this study involve recombinant DNA? No Yes, IBC approval required 1. Will the Company provide any equipment to be installed in University space? 7. Will the Study require identifiable human subject information to 8. Does this study involve human stem cells? No Yes, hSCRO letter required No Yes, please explain: be sent to Company? If yes, stem cells are: adult embryonic No Yes 2. Equipment provided will be a: 9. Will the Company provide any software to be installed on University or UPMC gift loan purchase equipment?
SECTION E-COMPLETE THIS SECTION IF HUMAN BIOLOGICAL MATERIALS USED SECTION N/A No Yes, please explain and specify equipment and location,: 1. Will your portion of the Study involve use 2. Will human biological materials collected from university 10. What other agreements/ funding/materials will be applicable to the Study? of human materials? human subjects be provided to the Company? {list all that apply, i.e, Sponsored Research Agreement, Government or Other Grant , CDA, No Yes, IRB approval letter required No Yes, IRB approval letter required License, MTA, Other Agreement (list Company/ies)} Please provide Institution Number/Project Number or specific details for each: Prospectively collected 3. Human biological materials being provided are: Previously collected under another research Prospectively collected
CRA Submission Form (9/2006; Revised 1/2011; Revised 10/2012, Revised 11/2013) University Principal Investigator (PI) Contact Information: Company/Institutions Involved in the Where will the University portion of the Study be performed? Name: Study (include all parties even if not a Title: party to the agreement): Will research use the Regional Biocontainment Laboratory (RBL)?
Email: No Yes, attach the applicable RBL approval letter. PI’s Departmental Affiliation & Administrative Contact for Agreement Follow-up: Company/Institution Legal Contact for Provide Study Title and also please attach a brief scope of work: (Not Office of Research Staff) negotiations: PI’s Department: PI’s Dept. ID: Contract Administrator: Phone/Email: Name: Email: Phone: SECTION A-ALL STUDIES MUST COMPLETE THIS SECTION SECTION B-FOR BASIC OR ANIMAL SECTION C-FOR STUDIES INVOLVING HUMAN SUBJECTS RESEARCH STUDIES SECTION N/A SECTION N/A project Previously collected under another research project
To the best of my knowledge, the answers to the questions are true, complete and accurate. I have read the referenced agreement and agree to comply with its terms and conditions. I am a University of Pittsburgh faculty member authorized to disclose or receive the information noted above and to conduct the research contemplated by the contract. Principal Investigator: Date:
CRA Submission Form (9/2006; Revised 1/2011; Revised 10/2012, Revised 11/2013)