13.5 ANSWERS TO EXERCISES
13.5 Exercise 1
1. a) propanoic acid b) propan-1-ol c) no reaction H H H H O H
H C C C H C C C OH OH H H H H H
d) propan-2-ol e) 2-hydroxymethylpropanenitrile OH H OH H C N CH3 C H C C C H
H H H CH3
f) butanoic acid g) methylpropanoic acid h) no reaction H H O H C C C H H H O OH H C C C H C C H H OH H H H H
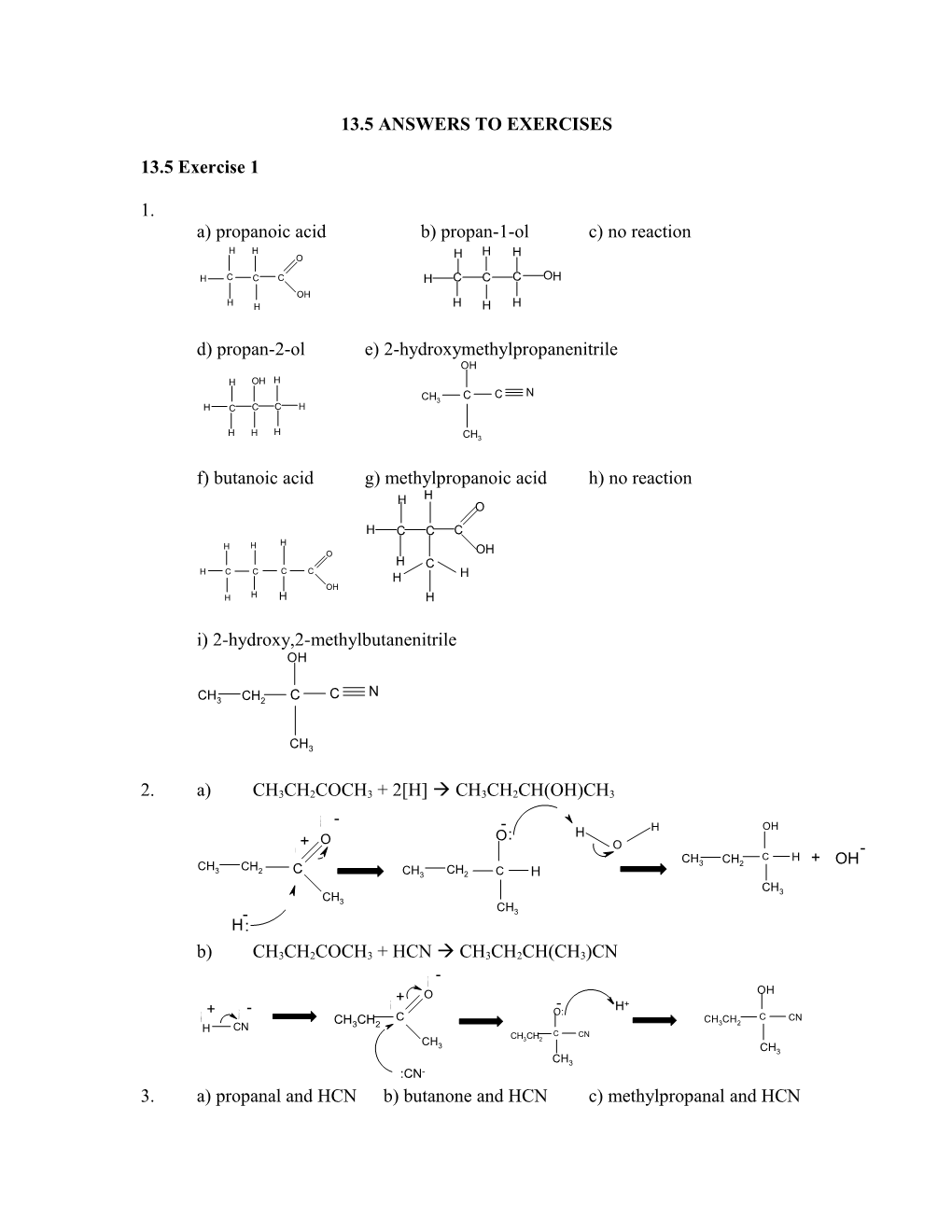
i) 2-hydroxy,2-methylbutanenitrile OH
N CH3 CH2 C C
CH3
2. a) CH3CH2COCH 3 + 2[H] CH3CH2CH(OH)CH3
- - OH H H O O: + O - C H CH3 CH2 + OH CH3 CH2 C CH3 CH2 C H CH CH 3 3 CH - 3 H:
b) CH3CH2COCH3 + HCN CH3CH2CH(CH3)CN - + O OH - + + - O: H C C CN CH CH CH3CH2 H CN 3 2 C CN CH3CH2 CH3 CH3 CH3 :CN- 3. a) propanal and HCN b) butanone and HCN c) methylpropanal and HCN 13.5 Exercise 2
- + 1. a) CH3CH2COOH + NaOH CH3CH2COO Na + H2O - + b) 2CH3CH2CH2COOH + Na2CO3 2CH3CH2CH2COO Na + CO2 + H2O c) HCOO-Na+ + HCl HCOOH + NaCl - + d) 2CH3COO Na + H2SO4 2CH3COOH + Na2SO4 - + e) 2CH3CH(CH3)COOH + Na2CO3 2CH3CH(CH3)COO Na + CO2 + H2O
2. a) CH3CH2COOH + CH3OH == CH3CH2COOCH3 + H2O organic product = methyl propanoate b) CH3COOH + CH3CH(OH)CH3 == CH3COOCH(CH3)CH3 + H2O organic product = methylethyl ethanoate c) HCOOH + CH3CH2CH2OH == HCOOCH2CH2CH3 + H2O organic product = propyl methanoate
d) CH3CH2CH2COOH + CH3CH2CH2CH2OH == CH3CH2CH2COOCH2CH2CH2CH3 + H2O organic product = butyl butanoate
3. a) O O OH H C + CH3 CH2 H C + H2O OH O CH2 CH3 b)
O O CH C + CH3 OH CH3 2 CH3 CH2 C + NaOH O-Na+ O CH3 c) OH O H O C CH3 H C C + CH C CH3 O CH CH 3 3 + H2O OH H H CH3 d) O O H C + NaOH H C CH OH O + CH3 CH2 2 CH2 CH2 CH3 O-Na+
4. a) H O H
O C H C C15H31 H C OH O O
+ 3H O H H O C C H 2 C OH HO C C H C 15 31 + 3 15 31 O H C OH C H C O C15H31 H
H
b)
H O H
O C H C C15H31 H C OH O O
C + H OH - H O C H 3NaOH C Na + O C C H C 15 31 + 3 15 31 O H C OH C H C O C15H31 H
H Reaction (a) takes place in the stomach The fatty acids are used in cell membranes The glycerol is used as an energy source Reaction (b) is carried out industrially The glycerol is used in pharmaceutical and cosmetic preparations The carboxylate salts are used as soaps
13.5 Exercise 3
1. a) CH3CH2COCl + NH3 CH3CH2CONH2 + HCl organic product: propanamide
b) (CH3CH2CH2CO)2O + CH3CH(OH)CH3 CH3CH2CH2COOCH(CH3)CH3 + CH3CH2CH2COOH organic products: methylethyl butanoate and butanoic acid c) HCOCl + CHCHNH HCONHCHCH + HCl organic product: N-ethyl methanamide
d) (CH3CO)2O + CH3CH(NH2)CH3 CH3CONHCH(CH3)CH3 + CH3COOH organic products: N-methylethylethanamide and ethanoic acid e) CH3CH(CH3)COCl + H2O CH3CH(CH)COOH + HCl organic products: methylpropanoic acid
2. a) H O
H C C OH + CH3 CH2 Cl H b) H H O H C C C + CH3 NH2 Cl H H c) OH
O C CH + CH3 3 CH3 CH2 C Cl H d) O CH NH H C + 3 CH2 2 Cl e) O OH O C CH3 C O H + O CH3 C O 3. a)
- O- + - O O O Cl.. CH3 C + HCl CH CH3 C 3 C Cl Cl CH3 C CH + N 3 .. + N H H N N CH3 H CH3 CH H H 3 H H b) nucleophilic addition-elimination c) acid anhydride is cheaper than acyl chloride the acid anhydride reaction is less violent the acid anhydride reaction does not produce toxic fumes of HCl