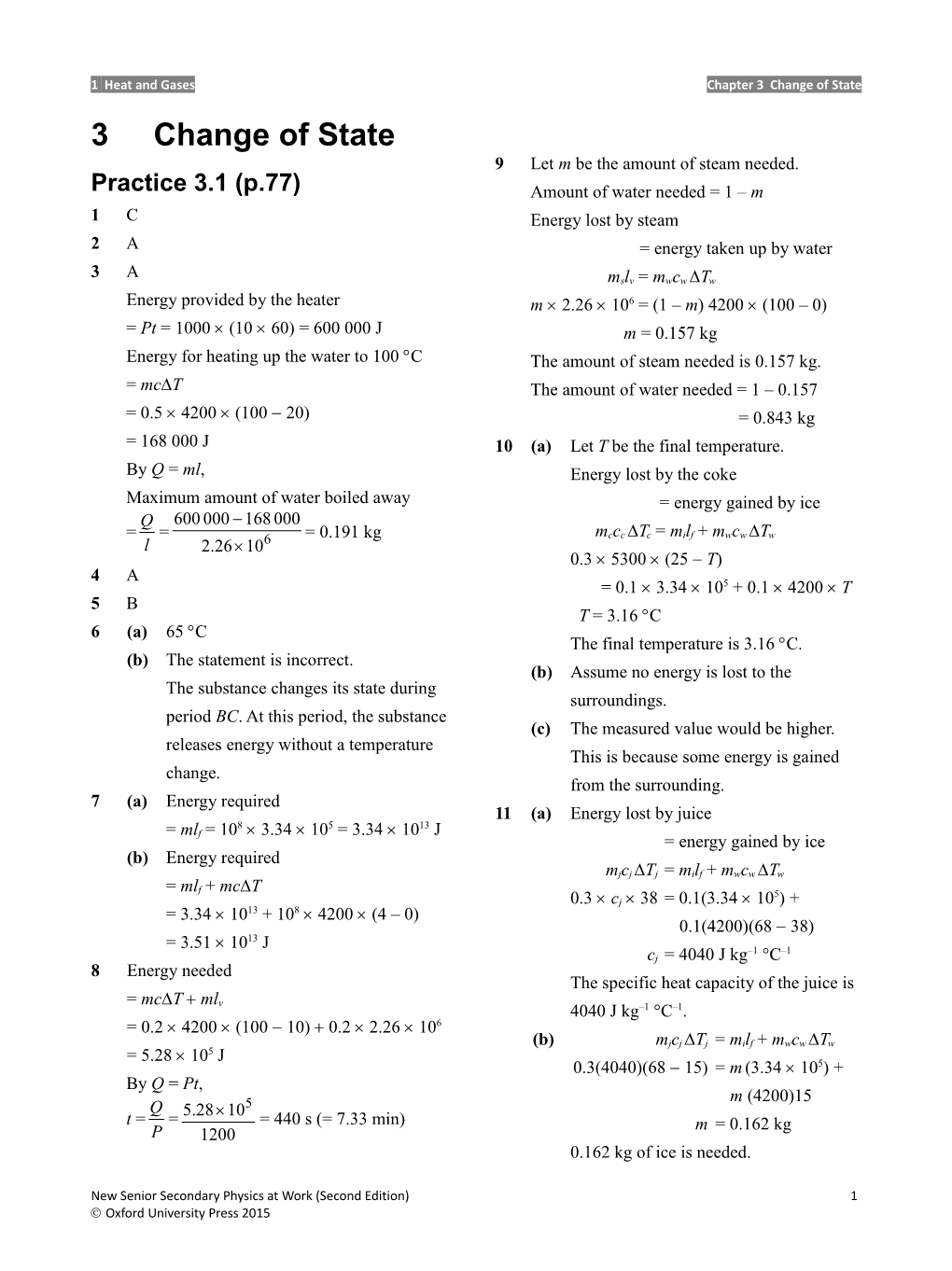
1 Heat and Gases Chapter 3 Change of State 3 Change of State 9 Let m be the amount of steam needed. Practice 3.1 (p.77) Amount of water needed = 1 – m 1 C Energy lost by steam 2 A = energy taken up by water 3 A mslv = mwcw Tw Energy provided by the heater m 2.26 106 = (1 – m) 4200 (100 – 0) = Pt = 1000 (10 60) = 600 000 J m = 0.157 kg Energy for heating up the water to 100 C The amount of steam needed is 0.157 kg. = mcT The amount of water needed = 1 – 0.157 = 0.5 4200 (100 20) = 0.843 kg = 168 000 J 10 (a) Let T be the final temperature. By Q = ml, Energy lost by the coke Maximum amount of water boiled away = energy gained by ice Q 600 000 168 000 = = = 0.191 kg mccc Tc = milf + mwcw Tw l 2.26 106 0.3 5300 (25 T) 4 A = 0.1 3.34 105 + 0.1 4200 T 5 B T = 3.16 C 6 (a) 65 C The final temperature is 3.16 C. (b) The statement is incorrect. (b) Assume no energy is lost to the The substance changes its state during surroundings. period BC. At this period, the substance (c) The measured value would be higher. releases energy without a temperature This is because some energy is gained change. from the surrounding. 7 (a) Energy required 11 (a) Energy lost by juice = ml = 108 3.34 105 = 3.34 1013 J f = energy gained by ice (b) Energy required mjcj Tj = milf + mwcw Tw = mlf + mcT 5 0.3 cj 38 = 0.1(3.34 10 ) + = 3.34 1013 + 108 4200 (4 – 0) 0.1(4200)(68 38) 13 = 3.51 10 J –1 –1 cj = 4040 J kg °C 8 Energy needed The specific heat capacity of the juice is = mcT ml v 4040 J kg–1 °C–1. = 0.2 4200 (100 10) 0.2 2.26 106 (b) mjcj Tj = milf + mwcw Tw 5 = 5.28 10 J 5 0.3(4040)(68 15) = m (3.34 10 ) + By Q = Pt, m (4200)15 Q 5.28 105 t = = = 440 s (= 7.33 min) m = 0.162 kg P 1200 0.162 kg of ice is needed.
New Senior Secondary Physics at Work (Second Edition) 1 Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
The specific latent heat of fusion of ice 12 (a) Energy for heating the water to steam is 3.19 105 J kg–1.
= mlv (b) The result in (a) is smaller than the = 0.3 2.26 106 standard value. = 678 000 J (c) The mixture absorbs energy from the Q Power of the steamer = surroundings. This makes the final l temperature T of the mixture higher. 678 000 = 0.3(4200)(20 T) 0.02(4200)T 5 60 lf = 0.02 = 2260 W =1.26 106 (6.72 104)T (b) The molecular KE remains unchanged T lf and the molecular PE increases. The value obtained in the experiment 13 Consider 0.3 kg of water at 50 C being mixed is smaller than the accepted value. with 0.2 kg ice at 0 C. 16 (a) None Energy absorbed when all the ice melts (b) c = ml = 0.2 3.34 105 = 6.68 104 J f (c) c This can cool down 0.3 kg of hot water by (d) lf 6.68104 53.0 C. However, since the (e) lf 0.3 4200 (f) lv, lf, c
initial temperature of the hot water is 50 C, (g) lv, lf, c the result will be ice-water mixture at 0 C. (h) None
On the other hand, the hot water cooled by the (i) lf 0-C water bath must have a final temperature higher than 0 C. Therefore, the ice can cool Practice 3.2 (p.86) the hot water to a lower temperature. 1 A 14 Energy lost by water = energy gained by ice 2 C
mwcw Tw = milf + mici Ti 3 C 5 0.25(4200)(80 0) = mi (3.34 10 ) + 4 Energy loss 6 6 mi (2060)[0 (4)] = mlv = 1.5 2.26 10 = 3.39 10 J
mi = 0.245 kg (= 245 g) 5 When we get out of a swimming pool, water The mass of ice needed is 0.245 kg. on our skin absorb s energy from our bodies 15 (a) Energy lost by water and evaporate s. Therefore, we lose energy and = energy gained by ice feel cold.
mwcw Tw = milf + mwcw Tw If it is windy, the evaporation rate, and
0.3(4200)(20 14) = 0.02lf + 0.02(4200)14 therefore the rate at which our bodies lose 5 1 lf = 3.19 10 J kg energy, w ill be higher. We would feel much cooler.
2 New Senior Secondary Physics at Work (Second Edition) Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
6 When water vapour meets a cool surface, it 9 Water vapour condenses to droplets on cold will condense on the surface and release surfaces. energy. Since the temperature is lower than the (a) The glasses are cooler than the freezing point, the droplets solidify to form surroundings when the person has just frost. got out of the car. Therefore, water 10 As glass A is half covered by a plastic sheet, vapour condenses on them. some water vapour that evaporates from the (b) The reason is similar to (a). The water hot water is trapped in the glass and the vapour in the steamy bathroom is at a density of vapour increases. Therefore, the higher temperature than the glasses. rate of evaporation is lower in glass A. Also, Also, the large amount of vapour water vapour condenses on the plastic sheet enhances the condensation. and drips back into the glass. As a result, the 7 (a) Assume that the latent heat of water level in glass A drops more slowly than vaporization is solely obtained from his that of glass B. body. 6 6 Q = mlv = 0.5 2.26 10 = 1.13 10 J Revision exercise 3 The maximum amount of energy Concept traps (p.89) removed is 1.13 106 J. 1 T (b) Rate of cooling by sweating Just after the ice and the hot water are mixed Q = together, the temperature of the mixture is t above 0 C. 1.13106 = 2 F 60 60 The liquid surfaces exposed to air are of the = 314 W same area in the two cases, so the rates of (c) Any two of the following: evaporation are the same. Temperature of the liquid Surface area of the liquid Multiple-choice questions (p.89) Density of vapour 3 B 8 (a) Energy taken away = mlv 4 A 6 = 0.2 2.26 10 5 A 5 = 4.52 10 J 6 B (b) By Q = mcT, 7 A Q decrease in temperature = 8 A mc 9 D 4.52 105 = 10 B 50 3500 11 A = 2.58 C 12 B
New Senior Secondary Physics at Work (Second Edition) 3 Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
13 C 21 (a) Total energy released by the water
Let T be the final temperature of the ‘mixture’. = mcwT + mlf + mciT 1M + 1M Energy lost by water = energy gained by ice = 1.5 4200 (30 – 0) + 1.5 5 mwcw Tw = milf + mwcw Tw 3.34 10 + 1.5 2060 [0 – (–5)] 1 4200 (20 T) = 0.5 3.34 105 + = 7.05 105 J 1A 0.5 4200 T (b) Average rate of energy loss by the water Q T = 13.2 C = 1M Since the final temperature should be between t 7.05105 0 C and 20 C, the result 13.2 C shows that = 5 60 60 not all the ice melts. Therefore, the final = 39.2 W 1A temperature of the ‘mixture’ is 0 C. 14 C 22 (a) Energy released by the water Let m be the mass of steam and km be the = mcT + mlf 1M 5 mass of ice. = 0.2 4200 30 + 0.2 3.34 10 Energy lost by steam = energy gained by ice = 92 000 J 1A (b) Since water has high specific heat mslv +mscw Tw = milf + micw Tw capacity and latent heat of fusion, 1A m 2.26 106 + m 4200 (100 50) it can release a large amount of energy = km 3.34 105 + km 4200 (50 0) k = 4.54 before it freezes. 1A The energy released will warm the 4.5 The ratio of the mass of ice to the mass of surroundings. 1A steam is 4.5 : 1. Therefore, spraying water on fruit trees 15 (HKCEE 2011 Paper 2 Q9) can protect fruits from freezing. 16 (HKCEE 2011 Paper 2 Q10) 23 (a) When 0.015 kg of steam at 100 C 17 (HKDSE 2013 Paper 1A Q1) condenses to water at 100 C, 18 (HKDSE 2013 Paper 1A Q2) energy released 6 19 (HKDSE 2014 Paper 1A Q2) = mlv = 0.015 2.26 10 = 33 900 J 1M Conventional questions (p.92) Power supplied by the steam cooker Q 20 (a) Melting point 1A = 1M t (b) Room temperature 1A 33 900 = (c) (i) The PE remains unchanged and the 60 average KE increases. 1A = 565 W 1A (ii) The PE increases and the average (b) Energy transferred to the dumplings KE remains unchanged. 1A = Pt 1M = 565 4 60 60%
4 New Senior Secondary Physics at Work (Second Edition) Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
= 81 400 J 1A Droplets accumulate and form larger 24 (a) Energy lost by the air droplets which then fall as rain. 1A = energy gained by ice (c) The condensation of vapour releases
macaT = milf 1M energy, 1A 70 1012 T = 1 3.34 105 so the surrounding air warms. 1A T = 4.71 C 1A 28 (a) (i) By Q = Pt = mcT, 1M The drop in temperature is 4.71 C. 350 t = 0.8 1300 (90 – 25) (b) He is correct. 1A t = 193 s 1A When snow melts, it absorbs latent heat The solid starts to melt at t = 193 s.
of fusion from its surroundings. 1A (ii) Pt = mlf 1M Therefore, the surrounding air loses 350 t = 0.8 6.10 104 energy and its temperature drops. 1A t = 139 s 1A 25 (a) Energy needed It takes 139 s to melt the solid
= mcT + mlv 1M + 1M completely. = 0.5 4200 (100 25) + temperature / C 0.5 2.26 106 (c) = 1.29 106 J 1A 90 (b) (b) (i) The energy required is larger than the result in (a). 1A (ii) Steam reaching the lid condenses to 25 time / s water droplets 1A 193 332 and may drip back to the pot. 1A (b) (Correct labelled axes with units) 1A 26 (a) By Q = Pt = mlv, 1M (Correct melting point) 1A 20 3 60 = 0.01 lv (Correct time when the solid starts to 5 –1 lv = 3.6 10 J kg 1A melt) 1A The specific latent heat of vaporization (Correct time when the solid melts 5 –1 of the liquid is 3.6 10 J kg . completely) 1A (b) Energy loss to the surroundings (c) (Slopes larger than those in the old 5 5 = (3.6 10 3.00 10 ) 0.01 1M curve) 1A = 600 J 1A (Shorter horizontal line) 1A 27 (a) Evaporation/vaporization 1A (Horizontal lines at the same height) 1A (b) The vapour is heated up by the sun and 29 (a) In heating the water, the balance reading rises. 1A remains unchanged at first. This shows The temperature is lower in higher that the water temperature is below the altitude. Therefore, the vapour boiling point and keeps increasing. 1A condenses back to water droplets. 1A
New Senior Secondary Physics at Work (Second Edition) 5 Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
The balance reading then drops. It shows 30 (a) (i) The air current brought by the fan that the boiling point is reached. Water takes the water molecules to other temperature keeps steady. 1A parts of the room. 1A Hence, it is easier for the water in the wick to evaporate. 1A
(ii) Yes, it is true. 1A As relative humidity increases, the rate of evaporation decreases. It is harder for the water to evaporate from the wick. 1A (b) I would feel cooler. 1A This is because the water in the wick (Temperature increases at first and absorbs energy from the surroundings to remains steady afterwards.) 1A vaporize. 1A (b) Since the beaker has more water, the 31 (HKCEE 2005 Paper 1 Q3) initial balance reading is larger. 1A 32 (HKCEE 2007 Paper 1 Q4) By Pt = mcT, it will take a longer time 33 (HKCEE 2009 Paper 1 Q3) for the heater to boil the water. Hence, 34 (HKCEE 2010 Paper 1 Q4) the horizontal line of the new curve is 35 (HKDSE 2012 Paper 1B Q1) longer and is above the old curve. 1A After the water boils, the rate of Experiment questions (p.96) vaporization of water depends on the 36 (a) The control set-up is used to determine power of the heater, which is unchanged. the amount of ice melted due to the Hence, the slope of the graph remains energy gain from the surroundings. 1A the same. 1A balance reading / kg Q (b) More ice melts. By lf = , 1A m
The value of lf obtained will be smaller. 1A (c) Her suggestion is incorrect. 1A
When measuring the value of lv, the water at 100 C would never gain energy time / s 0 from the surroundings to boil. The effect due to evaporation is negligible. As a (Longer horizontal line above the old result, the reading of the electronic curve, same slope) 1A balance in the control experiment would
6 New Senior Secondary Physics at Work (Second Edition) Oxford University Press 2015 1 Heat and Gases Chapter 3 Change of State
be unchanged. Hence it provides no useful information to reduce error. 1A 37 Put the crushed melting ice in the polystyrene
cup. Measure the mass m1 of ice in the cup using the electronic balance. 1A Insert the immersion heater completely to the cup of ice. Turn on the heater for a period of time. Measure this time interval using the stop-watch. 1A Remove the heater and any water in the cup.
Measure the mass m2 of ice remaining in the cup using the electronic balance. 1A Pt The latent heat of fusion of ice = . m1 m2 1A Possible source of error: Some energy is used to heat up the heater. 1A (Or other reasonable answers)
Physics in article (p.97) 38 (a) The humidity has to be low enough 1A for evaporation to take place effectively. 1A (b) The evaporative cooler increases the relative humidity in a room. 1A This makes sweat difficult to evaporate and results in discomfort. 1A (c) No, it is not. 1A A fan can only circulate air or speed up the evaporation of water on our skin. 1A (Accept other reasonable explanation.)
New Senior Secondary Physics at Work (Second Edition) 7 Oxford University Press 2015