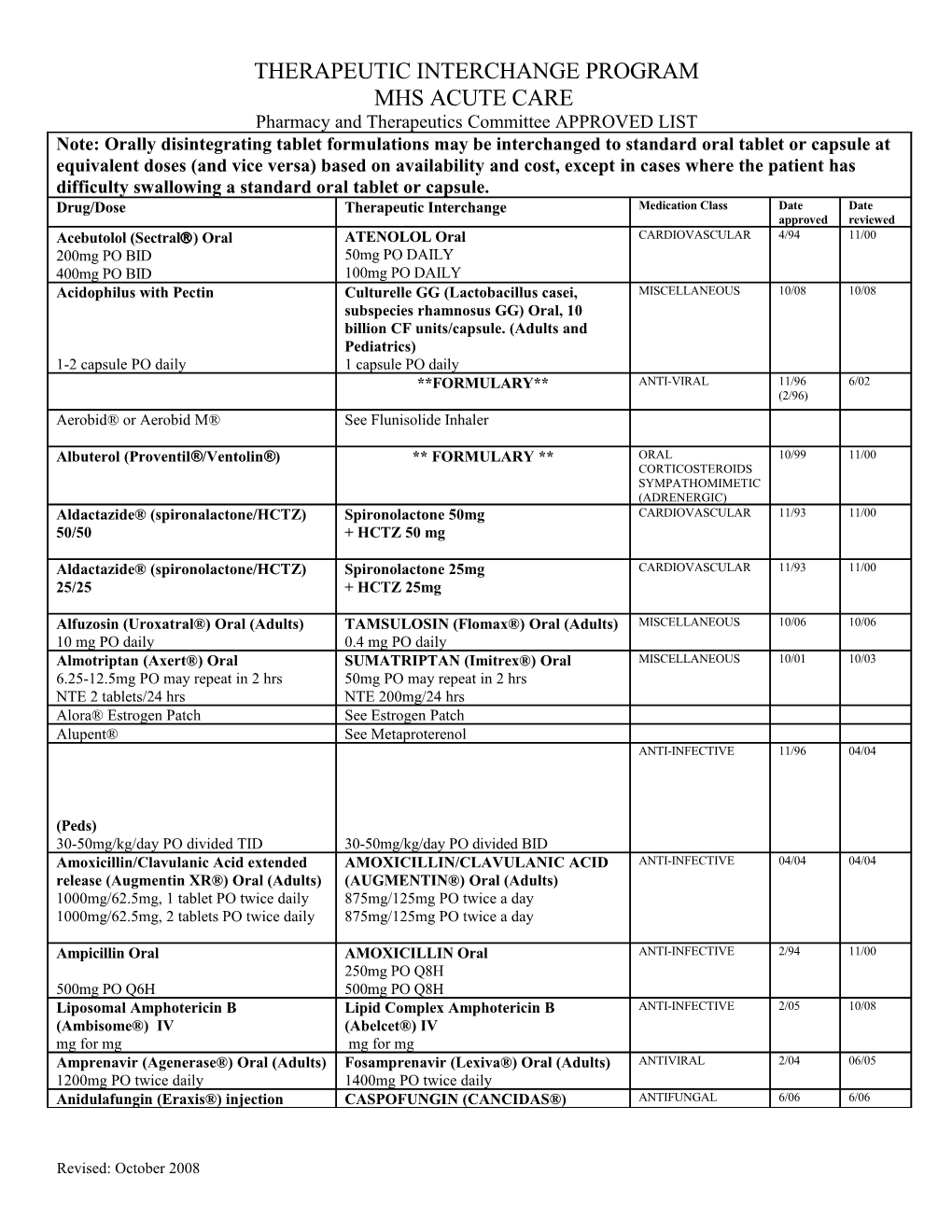
THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Acebutolol (Sectral) Oral ATENOLOL Oral CARDIOVASCULAR 4/94 11/00 200mg PO BID 50mg PO DAILY 400mg PO BID 100mg PO DAILY Acidophilus with Pectin Culturelle GG (Lactobacillus casei, MISCELLANEOUS 10/08 10/08 subspecies rhamnosus GG) Oral, 10 billion CF units/capsule. (Adults and Pediatrics) 1-2 capsule PO daily 1 capsule PO daily **FORMULARY** ANTI-VIRAL 11/96 6/02 (2/96) Aerobid® or Aerobid M® See Flunisolide Inhaler
Albuterol (Proventil®/Ventolin®) ** FORMULARY ** ORAL 10/99 11/00 CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Aldactazide® (spironalactone/HCTZ) Spironolactone 50mg CARDIOVASCULAR 11/93 11/00 50/50 + HCTZ 50 mg
Aldactazide® (spironolactone/HCTZ) Spironolactone 25mg CARDIOVASCULAR 11/93 11/00 25/25 + HCTZ 25mg
Alfuzosin (Uroxatral®) Oral (Adults) TAMSULOSIN (Flomax®) Oral (Adults) MISCELLANEOUS 10/06 10/06 10 mg PO daily 0.4 mg PO daily Almotriptan (Axert®) Oral SUMATRIPTAN (Imitrex®) Oral MISCELLANEOUS 10/01 10/03 6.25-12.5mg PO may repeat in 2 hrs 50mg PO may repeat in 2 hrs NTE 2 tablets/24 hrs NTE 200mg/24 hrs Alora® Estrogen Patch See Estrogen Patch Alupent® See Metaproterenol ANTI-INFECTIVE 11/96 04/04
(Peds) 30-50mg/kg/day PO divided TID 30-50mg/kg/day PO divided BID Amoxicillin/Clavulanic Acid extended AMOXICILLIN/CLAVULANIC ACID ANTI-INFECTIVE 04/04 04/04 release (Augmentin XR®) Oral (Adults) (AUGMENTIN®) Oral (Adults) 1000mg/62.5mg, 1 tablet PO twice daily 875mg/125mg PO twice a day 1000mg/62.5mg, 2 tablets PO twice daily 875mg/125mg PO twice a day
Ampicillin Oral AMOXICILLIN Oral ANTI-INFECTIVE 2/94 11/00 250mg PO Q8H 500mg PO Q6H 500mg PO Q8H Liposomal Amphotericin B Lipid Complex Amphotericin B ANTI-INFECTIVE 2/05 10/08 (Ambisome®) IV (Abelcet®) IV mg for mg mg for mg Amprenavir (Agenerase®) Oral (Adults) Fosamprenavir (Lexiva®) Oral (Adults) ANTIVIRAL 2/04 06/05 1200mg PO twice daily 1400mg PO twice daily Anidulafungin (Eraxis®) injection CASPOFUNGIN (CANCIDAS®) ANTIFUNGAL 6/06 6/06
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed INJECTION Invasive Candidiasis Anidulafungin 200mg IV on day one, Caspofungin 70mg IV on day one, followed followed by 100mg IV daily by 50mg IV daily
Esophageal Candidiasis Anidulafungin 100mg IV on day one, Caspofungin 50mg IV daily followed by 50mg IV daily Antacids MAALOX TC OR MYLANTA GASTROINTESTINAL 11/93 11/00
Antihistamine Decongestant Regular ACTIFED ANTIHISTAMINE/ 10/94 11/00 Release 1 TAB Q4-6H UP TO 4 TABS/DAY DECONGESTANT Tablet/capsule AntihistamineDecongestant Sustained DIMETAPP EXTENTABS ANTIHISTAMINE/ 10/94 11/00 Release Tablet/capsule 1 TAB Q12H DECONGESTANT
Arformoterol (Brovana) Adults Albuterol , Adults INHALENT 6/07 6/07 15 mcg SVN Q12H 2.5 mg SVN Q6H Aripiprazole (Abilify) Injection, (Adults) Ziprasidone (Geodon®) Injection (Adults) ANTIPSYCHOTIC 2/07 2/07 5.25mg IM every 2 hours 10mg IM every 2 hours 9.75mg IM every 2 hours 20mg every 4 hours 15mg IM every 2 hours 20mg every 2 hours Asmanex Twisthaler® See Mometasone furoate Atorvastatin (Lipitor) Oral SIMVASTATIN (Zocor®) Oral CARDIOVASCULAR 6/97 11/00, 10mg PO DAILY 11/08 10mg PO DAILY higher 20mg PO DAILY 20mg PO DAILY strengths 40mg PO DAILY 40mg PO DAILY 80mg PO DAILY 80mg PO DAILY limit the simvastatin dose per day to 20mg when used concomitantly with amiodarone Atripla® (efavirenz, emtricitabine, Efavirenz/Emtricitabine/Tenofovir, Oral ANTIVIRAL 2/07 2/07 tenofovir), Oral, (Adults) (Adults)
1 tablet (efavirenz 600mg, emtricitabine 1 tablet Efavirenz 600mg once daily + 200mg, tenofovir 300mg) once 1 tablet Emtricitabine 200mg once daily + daily 1 tablet Tenofovir 300mg once daily Avandaryl (Rosiglitazone/Glimepiride) ROSIGLITAZONE (Avandia®) + ANTIDIABETIC 10/06 10/06 Oral, Adults GLIMEPIRIDE (Amaryl®), Oral, Adults 4mg/1mg once daily 4mg + 1mg once daily 4mg/2mg once daily 4mg + 2mg once daily 4mg/2mg two tablets once daily 8mg + 4mg once daily (8mg/4mg total) 4mg/4mg once daily 4mg + 4mg once daily Note: Maximum daily dose is 8mg/4mg. Rosiglitazone/metformin (Avandamet®) ROSIGITAZONE (AVANDIA®) + ANTIDIABETIC 2/03 10/06 Oral, Adults METFORMIN (GLUCOPHAGE®), Oral,
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 1 mg/500 mg PO TWICE DAILY Adults (2 mg/1000 mg total daily) 2 mg (1/2 tab) + 500 mg PO TWICE DAILY 1 mg/500 mg, 2 tabs PO TWICE DAILY (2 mg/1000 mg total daily) (4 mg/2000 mg total daily) 2mg + 1000mg PO TWICE DAILY 2 mg/500 mg PO TWICE DAILY (4 mg/2000 mg total daily) (4 mg/1000 mg total daily) 2 mg + 500 mg PO TWICE DAILY 2mg/500 mg, 2 tabs PO TWICE DAILY (4 mg/1000 mg total daily) (8 mg/2000 mg total daily) 4 mg + 1000 mg PO TWICE DAILY 4 mg/500 mg PO TWICE DAILY (8 mg/2000 mg total daily) (8 mg/1000 mg total daily) 4 mg + 500 mg PO TWICE DAILY 2 mg/1000 mg PO TWICE DAILY (8 mg/1000 mg total daily) (4 mg/2000 mg total daily) 2 mg + 1000 mg PO TWICE DAILY 4 mg/1000 mg PO TWICE DAILY (4 mg/2000 mg total daily) (8 mg/2000 mg total daily) 4 mg + 1000 mg PO TWICE DAILY Maximum daily dose is 8 mg/2000 mg (8 mg/2000 mg total daily) Azatadine (Optimine) Oral CYPROHEPTADINE (Periactin®) Oral ANTIHISTAMINE 10/94 11/00 1-2mg PO Q12H 4mg PO Q8H Azmacort® See Triamcinolone Inhaler Beclomethasone 42mcg (Vanceril®) FLUTICASONE 44mcg (Flovent®) INHALENT/NASAL 2/00 10/05 (Peds) (Peds) CORTICOSTEROIDS (10/99) 2 puffs QID 2 puffs BID 4 puffs BID 2 puffs BID FLUTICASONE 110mcg (Flovent®) 4 puffs QID 2 puffs BID Beclomethasone(Vanceril,Beclovent) MOMETASONE FUROATE 220 mcg (Adults) (ASMANEX TWISTHALER) (Adults) 1 puff QID 1 puff BID 2 puffs QID 2 puffs BID
Beclomethasone 84mcg (Vanceril DS®) FLUTICASONE 44mcg (Flovent®) INHALENT/NASAL 2/00 10/05 (Peds) (Peds) CORTICOSTEROIDS (10/99) 2 puffs BID 2 puffs BID Beclomethasone 84 mcg (Vanceril DS) MOMETASONE 220mcg (ASMANEX (Adults) TWISTHALER®) (Adults) 1-2 puffs BID 1 puff DAILY 3-4 puffs BID 2 puffs DAILY Beclomethasone 40mcg (QVAR®) MOMETASONE 220mcg (ASMANEX INHALENT/NASAL 10/02 10/05 (Adults) TWISTHALER®) (Adults) CORTICOSTEROIDS any puff ordered, any frequency, eg. 1 puff 1 puff DAILY BID Beclomethasone 80mcg (QVAR®) MOMETASONE 220mcg (ASMANEX any puff ordered, any frequency, eg. 1 puff TWISTHALER®) (Adults) BID 2 puffs DAILY
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Beclomethasone Nasal Inhaler products FLUTICASONE (Flonase) Nasal INHALENT/NASAL 10/99 10/05 CORTICOSTEROIDS Vancenase Pockethaler Inhaler 2 sprays DAILY ADRENALS 1 spray BID-QID Vancenase AQ (DS) 1-2 sprays DAILY Beconase AQ 1 spray BID- 2 BID Vancenase /Beconase 1 spray BID – QID
Beclovent See Beclomethasone Inhaler INHALENT/NASAL CORTICOSTEROIDS
BENAZEPRIL (Lotensin) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 4/94, 10/02 5mg PO DAILY 5mg PO DAILY 10/01 10mg PO DAILY 10mg PO DAILY 20mg PO DAILY 20mg PO DAILY 40mg PO DAILY 40mg PO DAILY
Betaxolol (Kerlone) Oral ATENOLOL Oral CARDIOVASCULAR 4/94 11/00 5 mg PO DAILY 25mg PO DAILY 10mg PO DAILY 50mg PO DAILY 20mg PO DAILY 100mg PO DAILY
Bethanecol Oral Bethanecol Oral MISCELLANEOUS 10/00 11/00 10mg PO 25mg PO (max 50mg in 6H period)
Bidil® - Hydralazine 37.5mg/Isosorbide Hydralazine 37.5mg + Isosorbide CARDIOVASCULAR 2/06 2/06 Dinitrate 20mg Dinitrate 20mg 1 tablet PO BID 1 tablet of each product PO BID 1 tablet PO TID 1 tablet of each product PO TID 2 tablets PO BID 2 tablets of each product PO BID 2 tablets PO TID 2 tablets of each product PO TID Bisoprolol (Zebeta) Oral ATENOLOL Oral CARDIOVASCULAR 4/94 11/00 5mg PO DAILY 50mg PO DAILY 10mg PO DAILY 75mg PO DAILY CrCl 15-35mL/min max rec atenolol dose=50mg/day CrCl <15mL/min max rec atenolol dose=25mg/day Hemodialysis: 50mg atenolol following each dialysis
BISOPROLOL/HCTZ (Ziac®) Oral ***FORMULARY*** CARDIVASCULAR 10/01 10/01 2.5mg/6.25mg 5mg/6.25mg 10mg/6.25mg Bitolterol (Tornalate®) ALBUTEROL (Proventil®/Ventolin®) INHALENT/NASAL 10/99 11/00
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 1-2 inhalations Q8H 1-2 inhalations Q6H CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Brimonidine 0.15% (Alphagan P®) Brimonidine 0.2% (Generic) Ophthalmic ANTIGLAUCOMA 5/05 5/05 Ophthalmic Same regimen Any regimen Bromfenac (Duract®) Oral IBUPROFEN Oral (Adults) NSAIDS 5/05 5/05 25mg every 6-8 hours 600mg every 6-8 hours Max dose = 150mg/day Max dose = 3200mg/day Bromfenac (Xibrom) Ophthalmic FLURBIPROFEN (OCUFEN) OPHTHALMIC 6/06 6/06 Ophthalmic 1 drop 2 times/day 1 drop 4 times/day Brompheniramine (Dimetane®) Oral CHLORPHENIRAMINE Oral ANTIHISTAMINE 10/94 11/00 4mg PO Q4-6H 4mg PO Q4-6H Bronkometer® See Isoetharine
Budesonide 200mcg (Pulmicort®) (Peds) FLUTICASONE 110mcg (Flovent®) INHALENT/NASAL 2/00 10/05 (Peds) CORTICOSTEROIDS (10/99) ADRENALS 1 inhalation BID 2 puffs BID 2 inhalations BID 3 puffs BID Budesonide (Pulmicort ®) MOMETASONE 220mcg (ASMANEX (Adults) TWISTHALER®) (Adults) 1puff BID 1 puff DAILY 2 puffs BID 1 puff DAILY 3-4 puffs BID 2 puffs DAILY
Budesonide Flexhaler 90mcg/inh Mometasone furoate (Asmanex® INHALER/NASAL 6/07 6/07 220mcg/inh) (Adults) CORTICOSTEROIDS ADRENALS 2-4 puffs twice daily 1 puff once daily 6-8 puffs twice daily 2 puffs once daily Budesonide Flexhaler 180mcg/inh Mometasone furoate (Asmanex® INHALER/NASAL 6/07 6/07 220mcg/inh) (Adults) CORTICOSTEROIDS ADRENALS 1-2 puffs twice daily 1 puff once daily 3-4 puffs twice daily 2 puffs once daily Budesonide (Rhinocort®) Nasal Inhaler FLUTICASONE (Flonase) Nasal In- INHALENT/NASAL 10/99 10/05 2 sprays BID or CORTICOSTEROIDS haler ADRENALS 4 sprays DAILY 2 sprays DAILY
Bupropion extended release (Wellbutrin Bupropion Sustained release CENTRAL NERVOUS 02/04 02/04 XL®) Oral (Adults) (Wellbutrin SR®) Oral (Adults) SYSTEM AGENT 150 mg PO once a day 150 mg once a day 300 mg PO once a day 150 mg PO twice a day Calcitonin (Miacalcin®) 200 unit/dose, CALCITONIN (FORTICAL®) 200 ENDOCRINE 2/06 2/06 3.7mL unit/dose, 3.7mL
Any regimen Same regimen Calcium carbonate / Vitamin D Calcium carbonate 500 mg / Vitamin D DIETARY 10/02 10/02 combination products Oral (Adults) 200 I.U. Oral (Adults) SUPPLEMENT CaCO3 600mg /vitamin D 600 I.U. CaCO3 500mg with vitamin D 200 I.U.
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Any frequency Same frequency CaCO3 600mg/Vitamin D 200 I.U. CaCO3 500mg with vitamin D 200 I.U. Any frequency Same frequency CaCO3 600mg with Vitamin D 125 I.U. CaCO3 500mg with vitamin D 200 I.U. Any frequency Same frequency CaCO3 500mg with Vitamin D 125 I.U. CaCO3 500mg with vitamin D 200 I.U. Any frequency Same frequency CaCO3 375mg with Vitamin D 125 I.U. CaCO3 500mg with vitamin D 200 I.U. Any frequency Same frequency CaCO3 315mg with Vitamin D 200 I.U. CaCO3 500mg with vitamin D 200 I.U. Any frequency Same frequency CaCO3 250mg with Vitamin D 125 I.U. ½ tablet CaCO3 500mg/vitamin D 200 I.U. Any frequency Same frequency Other rarely ordered calcium/vitamin D Contact Physician combinations Candesartan (Atacand®) Oral (Adults) VALSARTAN (DIOVAN®) Oral (Adults) CARDIOVASCULAR 2/99 2/06 4mg PO DAILY 20mg PO QD 8mg PO DAILY 40mg PO QD 16mg PO DAILY 80mg PO QD 32mg PO DAILY 160mg PO QD CAPTOPRIL (Capoten®) Oral ***FORMULARY*** CARDIOVASCULAR 9/01
Carbinoxamine (Clistin®) Oral DIPHENHYDRAMINE Oral ANTIHISTAMINE 10/94 11/00 4-8mg PO Q6-8H 25mg PO Q6-8H Carteolol (Cartrol) Oral ATENOLOL Oral CARDIOVASCULAR 4/94 11/00 2.5mg PO DAILY 25mg PO DAILY 5mg PO DAILY 50mg PO DAILY Carvedilol phosphate ER (COREG CR), Carvedilol (COREG), Oral (Adults) CARDIOVASCULAR 6/07 6/07 Oral, (Adults) 10mg DAILY 3.125mg BID 20mg DAILY 6.25mg BID 40mg DAILY 12.5mg BID 80mg DAILY 25mg BID Cefaclor (Ceclor) Oral (Adults) CEFPODOXIME(Vantin)Oral (Adults) ANTI-INFECTIVE 6/95 11/00 250mg PO TID 100mg BID 500mgPO TID 200mg BID Cefaclor (Ceclor) Oral (Peds) CEFPROZIL (Cefzil®) Oral (Peds) 20-40mg/Kg/day PO divided Q8-12H 15mg/Kg/dose PO BID Cefadroxil (Duricef) Oral CEPHALEXIN (Keflex) Oral ANTI-INFECTIVE 2/94 11/00 500mg PO Q12H 250mg PO Q6H 1000mg PO Q12H 500mg PO Q6H Cefazolin (Ancef, Kefzol) IV CEFAZOLIN IV ANTI-INFECTIVE 1/94 11/00 IV Q6HR IV Q8H (11/93) Interchange during shortage of cefoxitin Interchange during shortage of cefoxitin ANTI-INFECTIVE 9/03 11/06 only! only! Adults Adults Treatment: Cefoxitin (Mefoxin) IV Contact Prescriber 1-2 g IV q 6-8h
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed
Surgical Prophylaxis: Cefoxitin CEFAZOLIN (Ancef®) IV (Mefoxin) IV 1-2 g IV Q8H 1-2 g IV q 6-8h PLUS METRONIDAZOLE (Flagyl®) IV 500mg IV Q8H
Surgical Prophylaxis: Cefoxitin CEFAZOLIN (Ancef®) IV (Mefoxin) IV 1-2 g IV single dose 1-2 g IV single dose PLUS METRONIDAZOLE (Flagyl®) IV 500mg IV single dose
Pediatrics (> 3 month): Pediatrics (>3 month): Treatment: Cefoxitin (Mefoxin) IV Contact Provider 80-160 mg/kg/day divided every 6-8 hr
Surgical Prophylaxis: Cefoxitin CEFAZOLIN (Ancef®) IV (Mefoxin) IV 25mg/kg Q8H 80-160 mg/kg/day divided every 6-8 hr PLUS METRONIDAZOLE (Flagyl®) IV 10mg/kg Q8H
Surgical Prophylaxis: Cefoxitin CEFAZOLIN (Ancef®) IV (Mefoxin) IV 30-40mg/kg single dose 25mg/kg single dose PLUS METRONIDAZOLE (Flagyl®) IV 10mg/kg single dose
Neonates Neonates Cefoxitin (Mefoxin) IV ANY Contact Prescriber INDICATION, Any regimen Ceftibuten (Cedax) Oral (Adults) CEFPODOXIME (Vantin)Oral(Adults) ANTI-INFECTIVE 10/96 11/00 40mg PO DAILY 200mg PO BID CEFPROZIL (Cefzil) Oral (Peds) 9mg/kg/day PO (Peds) 15mg/kg/dose PO BID Ceftizoxime (Cefizox®) IV CEFOTETAN (Cefotan®) IV ANTI-INFECTIVE 9/03 9/03
FOR INDICATION: Surgical FOR INDICATION: Surgical Prophylaxis, Gynecologic infections Prophylaxis, Gynecologic infections (including PID), skin & soft tissue, intra- (including PID), skin & soft tissue, intra- abdominal infections, and UTI abdominal infections, and UTI infections. infections. Adults: Adults: 1-2 g IV every 8-12 hours 1-2 g IV q12 hours 1-2 g IV single dose 1-2 g IV single dose (same gram equivalent) Pediatrics: Pediatrics (> 1 month): 150-200mg mg/kg/day divided every 6-8 40-80 mg/kg/day divided every 12 hours hours Neonates (< 1 month) Neonates (< 1 month) Contact provider Contact provider
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed
FOR INDICATION: Aspiration FOR INDICATION: Aspiration pneumonia pneumonia Any Dose Contact Provider: Adults: Consider ceftriaxone 1-2 g IV every 24 hours + clindamycin 600mg IV every 8 hours Pediatric (>1 month): Consider ceftriaxone 50-75mg/kg/day as a once daily dose + clindmaycin 20- 40mg/kg/day in 3-4 divided doses
FOR INDICATION: Meningitis FOR INDICATION: Meningitis Any Dose Contact Provider Adults: Consider ceftriaxone 2g IV every 12 hours Pediatrics (> 1 month): Consider ceftriaxone 100 mg/kg/day in 1-2 divided doses every 12-24 hours; max 2 g IV every 12 hours
FOR INDICATION: Unknown or FOR INDICATION: Unknown or Uncertain Uncertain Contact Provider Contact Provider Cefuroxime axetil (Ceftin) Oral CEFPODOXIME (Vantin) Oral ANTI-INFECTIVE 6/95 11/00 (Adults) (Adults) 250mg PO BID 100mg PO BID 500mg PO BID 200mg PO BID Celecoxib (Celebrex®) Oral (Adults) ***FORMULARY**** NSAIDS 10/99 10/04 Cephradine (Velosef) Oral CEPHALEXIN (Keflex) Oral ANTI-INFECTIVE 2/94 11/00 250mg PO Q6H 250mg PO Q6H 500mg PO Q6H 500mg PO Q6H Cetacaine Topical Anes. Spray Topical HURRICAINE SPRAY Topical TOPICAL 11/00 ANESTHETIC
Cetirizine (Zyrtec) Syrup ***FORMULARY Syrup (Peds)**** NON-SEDATING 6/00 6/03 Ages 2-5 yrs: 2.5-5mg PO DAILY ANTIHISTAMINES Ages 6-11 yrs: 5-10mg PO DAILY
Cetirizine (Zyrtec®) Oral (Adult) LORATADINE (CLARITIN®) (Adult) NON-SEDATING 6/00 6/03 5mg PO DAILY 10mg PO DAILY ANTIHISTAMINES (2/00) 10mg PO DAILY 10mg PO DAILY Cetirizine 5mg / Pseudoephedrine LORATADINE (CLARITIN) Oral NON-SEDATING 9/03 9/03 120mg (Adults) ANTIHISTAMINE / DECONGESTANT (Zyrtec-D 12 Hour™ ) Oral (Adults) 10mg PO once daily COMBINATION One tablet PO twice daily plus PSEUDOEPHEDRINE Oral (Adults)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 60mg PO four times daily Chlorzoxazone (Parafon F/DSC) Oral CYCLOBENZAPRINE (Flexeril) Oral MUSCULOSKELETAL 06/05 06/05 (Adults) (Adults) 500mg three to four times daily 10mg three times daily > 65 years, 5mg three times daily Cimetidine Oral (Tagamet®) RANITIDINE (Zantac®) ORAL GASTROINTESTINAL 9/95 1/01 100mg PO (Tagamet® HB) 75 mg PO (Zantac 75®) (2/94), 1/01 300mg PO Q6H 150mg PO Q12H 400mg PO QHS 150 mg PO QHS 800mg PO QHS 300 mg PO QHS CIPROFLOXACIN (Cipro) Oral ***FORMULARY*** ANTI-INFECTIVE 6/00 3/06 Ciprofloxacin extended release tablet CIPROFLOXACIN Oral ANTI-INFECTIVE 02/04 3/06 (Cipro® XR) 500mg PO daily 250mg PO twice daily 1000mg PO daily 500mg PO twice daily Ciprofloxacin (Cipro®) IV LEVOFLOXACIN (Levaquin®) IV ANTI-INFECTIVE 6/00 3/06 400mg IV Q8H or Q12H 750mg IV Q24H 200mg IVQ12H 500mg IV x 1, then 250mg IV daily Ciprofloxacin Ophthalmic ***FORMULARY*** ANTI-INFECTIVE 11/03 3/05 Ciprodex (ciprofloxacin Ciprofloxacin 0.3% Ophthalmic Solution ANTI- 5/06 10/08 + INFECTIVE/ANTI- 0.3%/dexamethasone 0.1%) Suspension INFLAMMATORY Dexamethasone 0.1% Ophthalmic Suspension Clemastine (Tavist®) Oral DIPHENHYDRAMINE Oral ANTIHISTAMINE 10/94 11/00 1.34mg PO Q12H 25mg PO Q6-8H 2.68mg PO BID-TID Climara® Estrogen Patch ***FORMULARY***
Clindamycin (Cleocin) IV (Adults) CLINDAMYCIN (Cleocin) IV (Adults) ANTI-INFECTIVE 2/94 11/00 600mg IV Q6H 600mg IV Q8H 900mg IV Q6H 900mg IV Q8H (Peds) (Peds) 10-15mg/kg/dose IV Q 6H 10-15mg/kg/dose IV Q 8H Cloxacillin (Tegopen) Oral DICLOXACILLIN (Dynapen) Oral ANTI-INFECTIVE 1/94 11/00 250mg PO Q6H 125mg not available. Contact Prescriber. (11/93) 500mg PO Q6H 250mg PO Q6H CYCLOBENZAPRINE (FLEXERIL®) ***FORMULARY*** MUSCULOSKELETAL 06/05 06/05 DARBEPOETIN (ARANESP®) DARBEPOETIN (ARANESP®) BLOOD MODIFIER 01/04 01/04 INJECTION (ADULTS) INJECTION (ADULTS) Reduce dose by half and administer weekly based on previously scheduled day (example) 200 mcg SC/IV every 2 weeks 100 mcg SC/IV weekly 300 mcg SC/IV every 2 weeks 150 mcg SC/IV weekly Darifenacin ER (Enablex®) Oral SOLFENACIN (VESICARE®) Oral MISCELLANEOUS 2/06 2/06 (Adults) (Adults) 7.5mg daily 5mg (1/2 x 10mg) daily 15mg daily 10mg daily
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Desloratidine (Clarinex®) Oral LORATADINE (CLARITIN®) Oral NON-SEDATING 6/02 6/03 (Adults) (Adults) ANTIHISTAMINE 5mg PO DAILY 10 mg PO DAILY
Dexchlorpheniramine (Polaramine®) CHLORPHENIRAMINE ANTIHISTAMINE 10/94 11/00 Oral Oral 2mg PO Q4-6H 4mg PO Q4-6H Diclofenac Sodium (Voltaren®) IBUPROFEN Oral (Adults) NSAIDS 5/05 5/05 25mg BID-Q6H 400mg Q8H-Q6H 50mg BID-Q8H 600mg Q6H 50mg Q6H 600mg Q6H 75mg BID 800mg Q8H
Diclofenac SR(VoltarenXR®) IBUPROFEN Oral (Adults) NSAIDS 5/05 5/05 100mg daily 400mg Q6H 200mg daily 600mg Q6H Diclofenac (Voltaren) Ophthalmic FLURBIPROFEN (OCUFEN) OPHTHALMIC 6/06 6/06 Ophthalmic 1 drop 4 times/day 1 drop 4 times/day Diflunisal (DoloBID®) IBUPROFEN Oral (Adults) NSAIDS 5/05 5/05 250 mg BID 400mg Q6H 500 mg BID 600mg Q6H Digoxin capsules (Lanoxicaps®) Oral DIGOXIN (Lanoxin®) Oral CARDIOVASCULAR 10/94 11/00 0.1mg PO 0.125mg PO 0.2mg PO 0.25 mg PO
Digoxin Immune Fab Ovine (Digibind®) Digoxin Immune Fab Ovine DigiFab® IV ANTIDOTE 6/02 6/02 IV Dosing based on weight and serum digoxin Dosing based on weight and serum digoxin concentration. concentration. Diltiazem CD (Cardizem® CD) Oral DILTIAZEM (Tiazac®) Oral CARDIOVASCULAR 3/98 11/00 Any dose, any frequency SAME DOSE AND FREQUENCY Dinoprostone intravaginal gel DINOPROSTONE VAGINAL INSERT OB-GYN 3/03 3/03 (compounded product) (CERVIDIL®) 5mg 10mg Dolasetron (Anzemet®) (Adults) Ondansetron Injection (Adults) ANTI-EMETIC 03/04 10/06 12.5mg IV 4mg IV 12.5mg IV may repeat once 4mg IV - no repeat dose 12.5mg IV any frequency 4mg IV same frequency 100-200mg IV 16 mg IV over 15 minutes >200mg IV 32mg IV over 30 minutes 100-200mg PO 24mg PO Dutaseride (Avodart®) Oral (Adults) FINASTERIDE (PROSCAR®) Oral MISCELLANEOUS 6/06 6/06 (Adults) 0.5mg PO daily 5 mg PO daily
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Duoneb (Ipratropium 0.5mg/Albuterol Albuterol 0.5% 0.5ml (2.5mg albuterol RESPIRATORY 10/01 10/01, 3mg (2.5mg albuterol base)) base) and Ipratropium 0.02% 2.5ml 8/03 3ml via SVN with any dosing interval (0.5mg) (total dose 3ml) via SVN with any dosing interval. Dyazide® (50/25) MAXZIDE-25 (37.5/25) CARDIOVASCULAR 11/93 11/00 ELA-MaxTM (lidocaine 4% liposomal) ***FORMULARY*** TOPICAL 5/01 5/01 topical cream ANESTHETIC Elase® See Fibrinolysin+Desoxyribonuclease ENZYMATIC 11/00 DEBRIDING AGENTS Eletriptan (Relpax®), Oral, Adults SUMATRIPTAN (IMITREX®), Oral , MISCELLANEOUS 2/03 10/03 Adults 20-40mg at onset and may repeat in 2 hrs 50mg PO may repeat in 2 hrs NTE 80mg/24hrs NTE 200mg/24hrs EMLA® (lidocaine 2.5% and prilocaine ELA-MaxTM (lidocaine 4% liposomal) TOPICAL 5/01 5/01 2.5%) topical cream topical cream ANESTHETIC ENALAPRIL (Vasotec®) Oral, Injection ***FORMULARY*** CARDIOVASCULAR 10/01 Enoxacin (Penetrex)Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 3/06 400mg PO Q12H 500mg PO Q24H (2/94) 200mg PO Q12H 250mg PO Q24H
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Enoxaparin (Lovenox®) Injection DALTEPARIN(Fragmin®) CARDIOVASCULAR 3/01 2/03 INJECTION General Surgery VTE prophylaxis General Surgery VTE prophylaxis 20 mg SC DAILY Moderate Risk: 2,500 units SC daily
30mg SC BID starting 8-12h postop High Risk: 2,500 units SC 6 hours postop x1, then 5,000 units SC DAILY (if LMWH to start postop day 1, then start 5000 units SC DAILY) -or- -or- 40mg SC, 1-2 hr preop and DAILY postop 5,000 units SC 8-12 hr preop, then 5,000 units DAILY
Orthopedic Surgery VTE prophylaxis Orthopedic Surgery VTE prophylaxis 30mg SC BID start 12-24h post surgery or 2,500 units SC 6 hours postop x1, then 40mg SC DAILY 5,000 units SC DAILY (if LMWH to start postop day 1, then start 5000 units SC DAILY)
Medical Prophylaxis Medical Prophylaxis 30mg SC BID or 40mg SC DAILY 5000 units SC daily
Treatment DVT/PE Treatment DVT/PE 1.5mg/kg SC DAILY or 1mg/kg SC BID For patients less than 125kg, dose the dalteparin based on TBW using: 200 units/kg SC DAILY For high-risk patients 100mg/kg SC BID may be considered (i.e. malignancy, iliofemoral thrombosis, recurrent DVT/PE). For patients >125kg, consult MD. We still recommend dosing based on TBW, however, the MD may prefer alternatives (i.e. 125kg max dose, heparin infusion).
Unstable Angina/Non-Q MI ***FORMULARY*** 1mg/kg SC BID
Prophylaxis – Morbidly Obese Patients ***FORMULARY*** in ICU 40 mg SC BID Epinephrine for inhalation (Peds) RACEMIC EPINEPHRINE for RESPIRATORY, 3/99 11/00 inhalation (Peds) PEDIATRICS 0.05ml/kg/dose (0.5ml max dose)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed EPOETIN INJECTION (ADULTS) DARBEPOETIN (ARANESP®) BLOOD MODIFIER 01/04 01/04 INJECTION (ADULTS) 40,000units SC/IV weekly 100 mcg SC/IV weekly 60,000units SC/IV weekly 150 mcg SC/IV weekly
Patient with anemia secondary to multiple NO INTERCHANGE myeloma, myelodysplastic syndrome, or Waldenstroms macroglobulinemia
Patients with anemia in critical care or due NO INTERCHANGE chronic renal failure Eprosartan (Teveten®) Oral (Adults) VALSARTAN (DIOVAN®) Oral ANGIOTENSIN II 2/00 2/06 (Adults) ANTAGONIST 400mg PO DAILY or 200mg PO BID 40mg PO QD or 20mg PO BID 600mg PO DAILY or 300mg PO BID 80mg PO QD or 40mg PO BID 800mg PO DAILY or 400mg PO BID 160mg PO QD or 80mg PO BID (Epzicom®) Abacavir/Lamivudine Oral ABACAVIR (Ziagen®) Oral (Adults) ANTIVIRAL 10/04 10/04 (Adults) 600mg PO once daily 600mg/300mg PO once daily Plus LAMIVUDINE (Epivir®) Oral (Adults) 300mg PO once daily Ertapenem (Invanz®) IV --NON-FORMULARY— ANTI-INFECTIVE 6/02 6/02 No therapeutic interchange. Suggest alternatives. It does not offer efficacy advantages over existing formulary broad-spectrum antibiotics. The drug in not indicated for pseudomonal, enterococcal or MRSA infections. Escitalopram (Lexapro®) Oral (Adults) CITALOPRAM (CELEXA®) Oral ANTIDEPRESSANT 2/05 2/05 (Adults) 5mg PO any schedule 10mg PO same schedule 10mg PO any schedule 20mg PO same schedule 20mg PO any schedule 40mg PO same schedule EsclimTM Estrogen Patch See Estrogen Patch HORMONE 3/01 3/01, REPLACEMENT 1/05 Esomeprazole (Nexium®) Oral (Adults) PANTOPRAZOLE (Protonix®) Oral GASTROINTESTINAL 6/01 6/05 (Adults) 20-40mg PO DAILY 40 mg PO DAILY
Esomeprazole (Nexium®) Oral (Adults) PANTOPRAZOLE (Protonix®) Oral GASTROINTESTINAL 6/01 6/05 (Adults) 20-40mg IV 40 mg IV
Estazolam (Prosom®) Oral TEMAZEPAM (Restoril®) Oral SEDATIVE/ 1/94 11/00 1mg PO 15mg PO HYPNOTIC (11/93)
Estraderm® Estrogen Patch See Estrogen Patch
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Estrogen Patch (17-β estradiol) Estrogen Patch (17-β estradiol) HORMONE 3/01 3/01, Alora® (twice weekly adminstration) Climara®(once weekly adminstration) REPLACEMENT 1/05 0.025 mg/day 0.025 mg/day 0.05 mg/day 0.05 mg/day 0.075 mg/day 0.075 mg/day 0.1mg/day 0.1 mg/day ESCLIM TM (twice weekly adminstration) Climara®(once weekly adminstration) 0.025 mg/day 0.025 mg/day 0.0375 mg/day 0.0375 mg/day 0.05 mg/day 0.05 mg/day 0.075 mg/day 0.075 mg/day 0.1 mg/day 0.1 mg/day Estraderm® (twice weekly admin.) Climara®(once weekly adminstration) 0.05 mg/day 0.05 mg/day 0.1 mg/day 0.1 mg/day Estrogen Patch (17-β estradiol) cont. Estrogen Patch (17-β estradiol) cont. Vivelle® (twice weekly adminstration) Climara®(once weekly adminstration) 0.025 mg/day 0.025 mg/day 0.0375 mg/day 0.0375 mg/day 0.05 mg/day 0.05 mg/day 0.075 mg/day 0.075 mg/day 0.1mg/day 0.1 mg/day Vivelle-DOT® (twice weekly admin.) Climara®(once weekly adminstration) 0.025 mg/day 0.025 mg/day 0.0375 mg/day 0.0375 mg/day 0.05 mg/day 0.05 mg/day 0.075 mg/day 0.075 mg/day 0.1 mg/day 0.1 mg/day Eszopiclone (Lunesta®) Oral (Adults) ZOLPIDEM (AMBIEN®) Oral (Adults) SEDATIVE/HYNOTIC 2/05 6/06 1 mg PO at bedtime 2.5mg PO at bedtime 2 mg PO at bedtime 5 mg PO at bedtime 3 mg PO at bedtime 10 mg PO at bedtime Etodolac (Lodine®) ***FORMULARY*** NSAIDS 5/05 5/05
Amlodipine/Valsartan (Exforge®), Amlodipine + Valsartan, Adults CARDIOVASCULAR 10/07 10/07 Adults 5 mg/160 mg Amlodipine 5 mg Valsartan 160 mg
5 mg/320 mg Amlodipine 5 mg Valsartan 320 mg
10 mg/160 mg Amlodipine 10 mg Valsartan 160 mg
10 mg/320 mg Amlodipine 10 mg Valsartan 320 mg Famciclovir Oral (Famvir®) ACYCLOVIR (Zovirax®) ORAL (Adults) ANTIVIRAL 6/02 6/02 (Adults)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 500mg PO Q8H or Q12H 800mg 5 times daily (CrCl >=25 ml/min)
500mg PO, other schedule: Acyclovir dosed appropriately for renal function as follows: 800mg PO Q8H (CrCl 10-25 ml/min) 800mg PO Q12H (CrCl <10 ml/min)
125mg PO Q12H (for genital herpes 200mg PO 5 times daily (CrCl >10ml/min) recurrence) 200mg PO Q12H (CrCl<10 ml/min)
250mg PO Q12H (for genital herpes 400mg PO Q12H (CrCl >10ml/min) suppression) 200mg PO Q12H (CrCl <10ml/min)
Oral, Pediatrics: Call prescriber and recommend acyclovir dosing based on appropriate indication Famotidine IV (Pepcid®) RANITIDINE (Zantac®) Oral GASTROINTESTINAL 3/94 1/01 20mg IV Q12H 150mg PO Q12H (When patient able to take (11/93), 1/01 PO medications) Famotidine Oral (Pepcid®) RANITIDINE (Zantac®) ORAL GASTROINTESTINAL 3/94, 9/95, 1/01 10mg PO (Pepcid® AC) 75 mg PO (Zantac 75®) 1/01 20mg PO Q12H 150mg PO Q12H 20mg PO QHS 150 mg PO QHS 40mg PO QHS 300 mg PO QHS Felodipine (Plendil®) Oral PROCARDIA XL Oral CARDIOVASCULAR 4/94 11/00 5mg PO DAILY 30mg PO DAILY 10mg PO DAILY 60mg PO DAILY
Fenoprofen (Nalfon®) IBUPROFEN Oral (Adults) NSAIDS 1/94 5/05 200mg Q8H-Q6H 200mg Q8H-Q6H (11/93) 300mg Q8H-Q6H 400mg Q8H-Q6H 600mg Q8H-Q6H 600mg Q8H-Q6H Fenofibrate (Tricor®) Oral (Adults) FENOFIBRATE Oral (Adults) CARDIOVASCULAR 2/05 10/07 48 mg PO any schedule 67 mg PO same schedule 145 mg PO any schedule 134mg PO same schedule FENOFIBRATE (Lofibra®) Oral FENOFIBRATE Oral (Adults) CARDIOVASCULAR 2/05 10/07 (Adults) 54mg PO any schedule 54 mg PO same schedule 67 mg PO any schedule 67mg PO same schedule 134mg PO any schedule 134 mg PO same schedule 160mg PO any scheule 160 mg PO same schedule 200mg PO any schedule 200 mg PO same schedule Fenofibrate (Antara®) Oral (Adults) FENOFIBRATE Oral (Adults) CARDIOVASCULAR 2/05 10/07 43 mg PO any schedule 67 mg PO same schedule 130 mg PO any schedule 200 mg PO same schedule FENOFIBRATE (Triglide®) Oral FENOFIBRATE Oral (Adults) CARDIOVASCULAR 2/05 10/07 (Adults) 50mg PO any schedule 67mg PO same schedule 160mg PO any schedule 200mg PO same schedule
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Fenofibrate (Lipofen®) Oral (Adults) FENOFIBRATE Oral (Adults) CARDIOVASCULAR 2/05 10/07 50 mg PO any schedule 67 mg PO same schedule 150 mg PO any schedule 134mg PO same schedule Fexofenadine (Allegra) (Peds) CETIRIZINE (Zyrtec) Syrup (Peds) NON-SEDATING 6/00 6/03 30mg PO DAILY 5-10mg PO DAILY ANTIHISTAMINE
Fexofenadine (Allegra) (Adult) LORATADINE (CLARITIN) (Adult) 180mg PO DAILY 10 mg PO DAILY 60mg PO BID 10 mg PO DAILY Fexofenadine 60mg / Pseudoephedrine LORATADINE (CLARITIN) Oral NON-SEDATING 9/03 9/03 120mg (Allegra-D®) Oral (Adults) (Adults) ANTIHISTAMINE / DECONGESTANT 1 tablet PO twice daily 10mg PO once daily COMBINATION plus PSEUDOEPHEDRINE Oral (Adults) 60mg PO four times daily
Fibrinolysin+Desoxyribonuclease UREA+PAPAIN (Accuzyme®) Unless for ENZYMATIC 2/98 11/00 (Elase®) Topical debridement of cancer patients DEBRIDING AGENTS with necrotic tissue then use Panafil®
Flunisolide 250mcg (AeroBid®) FLUTICASONE 44mcg (Flovent®) INHALENT/NASAL 2/00 10/02 (Peds) (Peds) CORTICOSTEROIDS (10/99) 2 puffs BID 2 puffs BID
Flunisolide (AeroBid®, AeroBid M®) MOMETASONE 220mcg (ASMANEX Inhaler (Adults) TWISTHALER®) (Adults) 1-2 puffs twice daily 1 puff DAILY 3-4 puffs twice daily 2 puffs DAILY
Flunisolide (Nasalide®, Nasarel) Nasal FLUTICASONE (Flonase) Nasal In- INHALENT/NASAL 10/99 10/05 Inhaler haler CORTICOSTEROIDS 2 sprays BID 2 sprays DAILY 2 sprays TID 2 sprays QID Flurazepam (Dalmane) TEMAZEPAM (RESTORIL) SEDATIVE/HYNOTIC 2/05 6/06 15mg PO at bedtime 15mg PO at bedtime 30mg PO at bedtime 30mg PO at bedtime Except for sleep clinic, pre-op use, pregnant and breast feeding mothers, and pediatric patients; then: Flurazepam (Dalmane) ZOLPIDEM (AMBIEN®) 15mg PO at bedtime 5 mg PO at bedtime 30mg PO at bedtime 10 mg PO at bedtime Flurbiprofen (Ansaid®) Oral IBUPROFEN Oral (Adults) NSAIDS 1/94 5/05 50mg PO any schedule 400mg PO same schedule (11/93) 100mg PO any schedule 800mg PO same schedule Fluticasone (Flovent) Nasal Inhaler ***FORMULARY** RESPIRATORY 10/99 10/05 INHALANT
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Fluticasone 44mcg, 110mcg, 220mcg ***FORMULARY (PEDS)** INHALENT/NASAL 10/99 10/05 (Flovent®) (Peds) CORTICOSTEROIDS
Fluticasone (Flovent®) (Adults) MOMETASONE (ASMANEX TWISTHALER®) (Adults) 44mcg, 110mcg: 1 puff twice daily 1 puff daily 220mcg: 1 puff twice daily 2 puffs daily
Fluticasone/Salmeterol (Advair HFA®) Fluticasone/Salmeterol (Advair Diskus®) INHALER/ 4/07 4/07 MDI (Pediatrics) (Pediatrics) CORTICOSTEROIDS 45/21 100/50 2 inhalations twice daily One inhalation twice daily 115/21 250/50 2 inhalations twice daily One inhalation twice daily 230/21 500/50 2 inhalations twice daily One inhalation twice daily Fluticasone/Salmeterol (Advair®) BUDESONIDE/FORMOTEROL INHALER/ 10/07 10/07 Inhaler (Adults) (SYMBICORT®) INHALER (ADULTS) CORTICOSTEROIDS 100/50mcg BID 80/4.5mcg 2 inhalations BID with spacer 250/50mcg BID 160/4.5mcg 2 inhalations BID with spacer 500/50mcg BID 160/4.5mcg 2 inhalations BID with spacer Fluvastatin (Lescol®) Oral SIMVASTATIN (Zocor®) Oral CARDIOVASCULAR 10/96 11/00 20mg PO QHS 5mg PO QHS (2/95) 40mg PO QHS 10mg PO QHS limit the simvastatin dose per day to 20mg when used concomitantly with amiodarone Fondaparinux (Arixtra®) Injection DALTEPARIN (Fragmin®) Injection CARDIOVASCULAR 2/02 2/02 2.5 mg SC DAILY 5000 units SC DAILY Formoterol (Foradil®) Aerolizer ***FORMULARY*** INHALENT/NASAL 6/03 6/03 CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Formoterol (Perforomist®) ALBUTEROL INHALENT/NASAL 10/08 10/08 20 mcg/2 ml SVN twice daily 2.5mg/3ml SVN every 6 hours CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Fosinopril (Monopril®) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 10/01 10/02 10mg PO DAILY 5mg PO DAILY 20mg PO DAILY 10mg PO DAILY 40mg PO DAILY 20mg PO DAILY Frovatriptan (Frova®) Oral SUMATRIPTAN (Imitrex®) Oral MISCELLANEOUS 2/02 10/03 2.5mg at onset and may repeat in 2hrs 25mg PO may repeat in 2 hrs NTE 7.5mg/24hrs NTE 200mg/24hrs Gatifloxacin (Tequin®) Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 6/00 3/06 400mg PO Q24H 500mg PO Q24H ABO sub- committee 200mg PO Q24H 250mg PO Q24H Gatifloxacin (Tequin®) IV LEVOFLOXACIN (Levaquin®) IV ANTI-INFECTIVE 6/00 3/06 400mg IV Q24H 500mg IV Q24H 200mg IV Q24H 250mg IV Q24H
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Glucovance® See Glyburide/Metformin Glyburide (Glynase® PRES-TAB) Oral GLYBURIDE (MICRONASE) Oral ANTIDIABETIC 2/94 11/00 1.5mg PO DAILY 2.5mg PO DAILY 3mg PO DAILY 5mg PO DAILY 4.5mg PO DAILY 7.5mg PO DAILY 6mg PO DAILY 10mg PO DAILY 3mg PO BID 5mg PO BID 4.5mg PO BID 7.5mg PO BID 6mg PO BID 10mg PO BID Glyburide/metformin (Glucovance®) Glyburide + metformin Oral, Adults ANTIDIABETIC 6/01 10/06 Oral, Adults 1.25mg/250mg once daily 1.25mg + 250mg once daily 1.25mg/250mg twice daily 1.25mg + 250mg twice daily 2.5mg/500mg twice daily 2.5mg + 500mg twice daily 5mg/500mg twice daily 5mg + 500mg twice daily Three tablets of 5mg/500mg daily Three tablets of 5mg + three tablets of (15mg/1500mg total daily) 500mg daily with same schedule as combination product Four tablets of 5mg/500mg daily Four tablets of 5mg + four tablets of 500mg (20mg/2000mg total daily) daily with same schedule as combination Note: Maximum recommended total daily product dose is 20mg/2000mg GoLytely® ***FORMULARY*** MISCELLANEOUS 2/06 2/06
GoLytely® Intolerant 4 bisacodyl tablets, wait 1-6hrs till 1st bowel movement, then take 8oz of GoLytely® every 10 min. up to 2 liters. Granisetron (Kytril®) IV (Adults) Ondansetron (Adults) ANTIEMETIC 03/04 4/07 1mg IV 16mg IV over 15 minutes 2mg Orally 24mg orally Granisetron (Kytril®) IV/Oral (Peds) Ondansetron (Peds Chemo – Inpatients ANTIEMETIC 03/04 4/07 only) Switch per Pediatric Antiemetic order Guaifenesin extended release 600 mg Guaifenesin syrup 100 mg/5 mL OR 200 MISCELLANEOUS 6/03 6/03 tablet (Mucinex®) mg/10 Ml
Adults: Adults: 1 tablet BID 200 mg Q4H while awake 2 tablets BID 400 mg Q4H while awake (maximum dose 2400 mg/day) (maximum dose 2400 mg/day)
Pediatrics: Pediatrics: 12 years and older: same as adult 12 years and older: same as adult
6-12 years: Guaifenesin extended release 6-12 years: Guaifenesin 200 mg every four 600 mg every 12 hours (maximum dose hours (maximum dose 1200 mg/day) 1200 mg/day)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 2-5 years: Guaifenesin extended release 2-5 years: Guaifenesin 100 mg every four 300 mg every 12 hours (maximum dose hours (maximum dose 600 mg/day) 600 mg/day)
Less than 2 years: 12 mg/kg/day in 6 Less than 2 years: 12 mg/kg/day in 6 divided doses divided doses HalfLytely® GoLytely® MISCELLANEOUS 2/06 2/06 2Liters plus 4 Bisacodyl tablets 4 Liters IBUPROFEN (Motrin®, Advil®) ** FORMULARY** NSAIDS 1/94 5/05 IMIPENEM (Primaxin) IV Doripenem (Doribax®) IV ANTI-INFECTIVE 9/08 9/08 ***DURING SHORTAGE ONLY*** ***DURING SHORTAGE ONLY*** (Adults) (Adults) 500mg IV Q6H (CrCl>50) 500mg IV Q8H (CrCl>50) 500mg IV Q8H (CrCl>50) 500mg IV Q8H (CrCl>50) 500mg IV Q8H (CrCl 30-50) 250mg IV Q8H (CrCl 30-50) 500mg IV Q12H (CrCl 10-30) 250mg IV Q12H (CrCl 10-30) 250mg IV Q12H 250mg IV Q12H
Pediatrics, NICU: Usual dose: Interchange to Meropenem, contact physician for order. Suggested Dose is:
Imipenem 15-25 mg/kg/dose IV Q6H Meropenem 10-40mg/kg/dose IV Q8H INDOMETHACIN (Indocin®) ** FORMULARY** NSAIDS 1/94 5/05 INSULIN DETEMIR, 100 units/mL ** FORMULARY** ANTIDIABETIC 6/06 6/06 1 unit INSULIN GLARGINE, 100 units/Ml ***FORMULARY*** ANTIDIABETIC 6/01 6/06 1 unit Insulin glulisine (Apidra®) INSULIN ASPART (NOVOLOG®) ANTIDIABETIC 6/04 6/04 100 units/mL 100 units/mL 1 unit 1 unit Insulin lispro (Humalog®) INSULIN ASPART (NOVOLOG®) ANTIDIABETIC 6/02 9/02 100 units/mL 100 units/mL 1 unit 1 unit Ipratropium (Atrovent) Inhaler TIOTROPIUM (SPIRIVA) RESPIRATORY 6/04 6/04 (Adults) HANDIHALER (Adults) Any number of inhalations, any frequency Inhale the contents of one capsule (18mcg) once daily Ipratropium/albuterol (Combivent) TIOTROPIUM (SPIRIVA) RESPIRATORY 6/04 6/04 Inhaler (Adults) HANDIHALER (Adults) plus ALBUTEROL INHALER (in non-ventilated patients only) (in non-ventilated patients only) 2 puffs 4 times daily Inhale the contents of one tiotropium capsule (18mcg) daily Plus Albuterol Inhaler, 2 puffs, 4 times daily
Irbesartan (Avapro®) Oral VALSARTAN (DIOVAN®) Oral ANGIOTENSIN II 2/00 2/06 37.5mg PO DAILY 20mg PO DAILY ANTAGONIST 75mg PO DAILY 40mg PO DAILY
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 150mg PO DAILY 80mg PO DAILY 300mg PO DAILY 160mg PO DAILY Isoetharine (Bronkometer®) ALBUTEROL (Proventil®/Ventolin®) INHALENT/NASAL 10/99 11/00 1-2 inhalations Q 4-6H 1-2 inhalations Q4-6H CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Isosorbide Mononitrate ISMO IMDUR CARDIOVASCULAR 11/95 11/00 10-20mg BID 30-60mg DAILY (11/93) Isradipine (Dynacirc®) Oral PROCARDIA XL Oral CARDIOVASCULAR 4/94 11/00 2.5mg PO BID 30mg PO DAILY 5mg PO BID 60mg PO DAILY Ketoprofen (Orudis®) IBUPROFEN NSAIDS 1/94 5/05 25mg Q8H-Q6H 400mg Q8H-Q6H (11/93) 50mg Q8H-Q6H 600mg Q8H-Q6H 75mg Q8H-Q6H 800mg Q8H-Q6H Ketorolac (Toradol®) Oral (Adults) IBUPROFEN Oral (Adults) NSAIDS 9/01 5/05 10 mg PO Q4-6H 600 mg PO Q6H (Pediatrics) (Pediatrics) Ketorolac any dose IBUPROFEN 10 mg/kg PO Q6-8H not to exceed 600 mg PO Q6H Ketorolac (Acular) Ophthalmic FLURBIPROFEN (OCUFEN) OPHTHALMIC 6/06 6/06 Ophthalmic 1 drop 4 times/day 1 drop 4 times/day Lactinex (Lactobacillus acidophilus and Culturelle GG (Lactobacillus casei, MISCELLANEOUS 11/06 10/08 Lactobacillus bulgaricus) Oral, 100 subspecies rhamnosus GG) Oral, 10 million CF units/packet (Adults and billion CF units/capsule. (Adults and Pediatrics) Pediatrics)
3 packets or greater per day 1 capsule orally twice daily (may be opened and mixed in liquid or applesauce)
1-2 packets orally per day 1 capsule orally once daily (may be opened and mixed in liquid or applesauce)
3-4 tablets PO TID-QID 1 capsule orally once daily (may be opened and mixed in liquid or applesauce) Lansoprazole (Prevacid) Oral (Adults) PANTOPRAZOLE (Protonix) Oral GASTROINTESTINAL 10/00 11/00 (Adults) (10/99) 15mg – 30mg PO DAILY 40mg PO DAILY 30mg PO BID 40mg PO BID
Lansoprazole (Prevacid) IV (Adults) PANTOPRAZOLE (Protonix) IV GASTROINTESTINAL 10/04 10/04 (Adults) 30mg IV any schedule 40mg IV same schedule 60mg IV any schedule 80mg IV same schedule
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Levalbuterol (Adults) and (Peds) ***FORMULARY*** RESPIRATORY 10/99 2/05 The literature is inconsistent as to the advantange of levalbuterol over albuterol. Pharmacist should contact prescriber, especially after 24 hours to see if patient may switch to albuterol, unless patient has a history of albuterol intolerance. If no intolerance and patient may switch after 24 hours, use the following: Any levalbuterol dose Pediatrics: <20kg: Albuterol SVN 2.5mg/dose >20kg: Albuterol SVN 5mg/dose Adults: Albuterol SVN 2.5mg/dose Levocetirazine (Xyzal™) 5mg tab LORATADINE (Claritin®) 10mg tab NON-SEDATING 6/08 6/08 (Adults) (Adults) ANTIHISTAMINES 5mg once daily 10mg once daily Levocetirazine (Xyzal™) 0.5mg/mL oral CETIRIZINE (Zyrtec) 1mg/mL Syrup NON-SEDATING 6/08 6/08 solution (Pediatrics) (Pediatrics) ANTIHISTAMINES 2.5mg once daily 2.5mg once daily 5mg once daily 5mg once daily Levofloxacin (Levaquin®) Oral ***FORMULARY*** ANTI-INFECTIVE 6/00 3/06 500mg PO Q24H ABO sub- committee 250mg PO Q24H 250mg PO Q48H Levofloxacin (Levaquin®) IV ***FORMULARY*** ANTI-INFECTIVE 6/00 3/06 500mg IV Q24H ABO sub- committee 250mg IV Q24H 250mg IV Q48H Lidocaine 5% patch (Lidoderm) Lidocaine 5% patch (Lidoderm) MISCELLANEOUS 2/08 2/08 2 patches daily 1 patch daily 3 patches daily 1 patch daily Lindane, (Kwell®) topical Permethrin (Nix®), topical MISCELLANEOUS 11/05 11/05 (Adults, Pediatrics) (Adults, Pediatrics) 1% Shampoo 1% lotion 1% lotion 5% cream
Lisinopril (Zestril®, Prinavil®) Oral ***FORMULARY*** CARDIOVASCULAR ___, 10/01 10/02 (Adults)
Lomefloxacin (Maxaquin®) Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 3/06 400mg PO DAILY 500mg PO Q24H (2/94) Loracarbef (Lorabid®) Oral (Peds) CEFPROZIL (Cefzil) Oral (Peds) ANTI-INFECTIVE 2/95 11/00 15-30mg/kg/day PO divided BID 30mg/kg/day PO divided BID
Loratadine (Claritin®) Oral (Peds) CETIRIZINE (Zytec) syrup (Peds) NON-SEDATING 6/00 6/03 5-10mg PO DAILY 5-10mg PO DAILY ANTIHISTAMINES (2/00)
Loratadine (Claritin®) Oral (Adult) ***FORMULARY***
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed
Loratadine 10mg / Pseudoephedrine LORATADINE (CLARITIN) Oral NON-SEDATING 9/03 9/03 240mg (Adults) ANTIHISTAMINE / DECONGESTANT (Claritin-D®) 24 hour) Oral (Adults) 10mg PO once daily COMBINANTION 1 tablet PO once daily plus ------OR------PSEUDOEPHEDRINE Oral (Adults) Loratadine 5 mg / Pseudoephedrine 60mg PO four times daily 120mg (Claritin-D®) 12 hour) Oral (Adults) 1 tablet PO twice daily Losartan (Cozaar®) Oral VALSARTAN (DIOVAN®) Oral CARDIOVASCULAR 2/99 2/06 25mg PO DAILY 40mg PO DAILY 50mg PO DAILY 80mg PO DAILY 100mg PO DAILY 160mg PO DAILY Lovastatin (Mevacor®) Oral SIMVASTATIN (Zocor®) Oral CARDIOVASCULAR 10/96 11/00 20mg PO DAILY 10mg PO DAILY (2/95) 40mg PO DAILY 20mg PO DAILY limit the simvastatin dose per day to 20mg when used concomitantly with amiodarone Magnesium Gluconate or Magnesium MAGNESIUM OXIDE Oral Tablets MISCELLANEOUS 10/01 10/01 Chloride (Slow-Mag®) Oral Tablets 27-256mg elemental magnesium DAILY 240mg elemental magnesium DAILY in any dose frequency Magnesium Gluconate Liquid ***FORMULARY*** MISCELLANEOUS 10/01 10/01 Magnesium Oxide Oral Tablets ***FORMULARY*** MISCELLANEOUS 10/01 10/01 Maxair® See Pirbuterol Meclofenamate (Meclomen®) IBUPROFEN Oral (Adults) 50mg Q8H-Q6H 400mg Q8H-Q6H 100mg Q8H 600mg Q6H 100mg Q6H 800mg Q6H Mefenamic acid (Ponstel®) IBUPROFEN Oral (Adults) 250mg Q6H 400mg Q6H Megestrol ES (Megace ES®) MEGESTROL ACETATE MISCELLANEOUS 10/06 10/06 625mg/5ml 800 mg/20mL Meloxicam (Mobic®) Oral (Adults) CELECOXIB (Celebrex®) Oral (Adults) ANALGESIC 6/02 10/04 7.5mg PO once daily 200mg PO once daily 15mg PO once daily 200mg PO twice daily *If sulfa allergy, contact physician Meropenem (Merrem®) IV IMIPENEM (Primaxin) IV ANTI-INFECTIVE 11/00, 1GM IV Q8H 9/08 500mg IV Q8H (CrCl>30) shortage 1GM IV Q12H 500mg IV Q8H (CrCl>30) 500mg IV Q12H (CrCl 5-30) 500mg IV Q12H 500mg IV Q12H 500mg IV Q24H 250mg IV Q12H
OTHER DOSES (includes Peds, NICU is CONTACT PHYSICIAN case-by-case)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed ***DURING SHORTAGE ONLY*** ***DURING SHORTAGE ONLY*** (Adults) Doripenem (Doribax®) IV(Adults) 1GM IV Q8H 500mg IV Q8H (CrCl>50) 1GM IV Q12H (CrCl 30-50) 250mg IV Q8H (CrCl 30-50) 500mg IV Q12H (CrCl 10-30) 250mg IV Q12H (CrCl 10-30) 500mg IV Q24H 250mg IV Q12H
Pediatrics, NICU Q12H Do not interchange. Metaglip® (glipizide/metformin) Oral, Glipizide (Glucotrol®) + Metformin ANTIDIABETIC 2/03 10/06 Adults (Glucophage®), Oral, Adults 2.5 mg/250 mg PO daily 5 mg (1/2 tab) + 500 mg (1/2 tab) PO daily (2.5 mg/250 mg total daily) (2.5 mg/250 mg total daily) 2.5 mg/250 mg PO BID 5 mg (1/2 tab) + 500 mg (1/2 tab) PO BID (5 mg/500 mg total daily) (5 mg/500 mg total daily) 2.5 mg/500 mg PO daily 5 mg (1/2 tab) + 500 mg PO daily (2.5 mg/500 mg total daily) (2.5 mg/500 mg total daily) 2.5mg/500 mg PO BID 5 mg (1/2 tab) + 500 mg PO BID (5 mg/1000 mg total daily) (5 mg/1000 mg total daily) 2.5 mg/500 mg, 2 tabs PO BID 5 mg + 1000 mg PO BID (10 mg/2000 mg total daily) (10 mg/2000 mg total daily) 5 mg/500 mg PO daily 5 mg + 500 mg PO daily (5 mg/500 mg total daily) (5 mg/500 mg total daily) 5 mg/500 mg PO BID 5 mg + 500 mg PO BID (10 mg/1000 mg total daily) (10 mg/1000 mg total daily) 5 mg/500 mg, 2 tabs PO BID 10 mg +1000 mg PO BID (20 mg/2000 mg total daily) (20 mg/2000 mg total daily) Maximum daily dose is 20 mg/2000 mg Maximum daily dose is 20 mg/2000 mg Metaproterenol (Alupent®) ALBUTEROL (Proventil®/Ventolin®) INHALENT/NASAL 10/99 11/00 1-2 inhalations Q 3-4H 1-2 inhalations Q4-6H CORTICOSTEROIDS SYMPATHOMIMETIC (same inhalations, decreased frequency) (ADRENERGIC)
Metaxalone (Skelaxin®) Oral (Adults) CYCLOBENZAPRINE (FLEXERIL®) MUSCULOSKELETA 06/05 06/05 Oral (Adults) L 800mg three to four times daily 10mg three times daily > 65 years, 5mg three times daily
Metformin XR (Glucophage XR®) Oral METFORMIN generic Oral ANTIDIABETIC 6/01 10/02 Any dose ordered (ie. 1000mg PO DAILY) Split the XR dose into equivalent dosing BID (ie. 500mg PO BID)
Any dose ordered BID up to 2000mg total Same dose BID (ie., 1000mg PO BID) dose per day (ie., 1000mg PO BID)
2550mg total dose per day in divided doses 850mg PO TID Methdilazine (Tacaryl®) Oral PROMETHAZINE Oral ANTIHISTAMINE 10/94 11/00 8mg PO Q6-12H 12.5-25mg PO Q6-24H Methylcellulose (Citrucel®) Psyllium (Metamucil®) MISCELLANEOUS 9/08 9/08 Any number of packets any regimen Same number of packets, same regimen
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed i.e. 1 packet twice daily i.e. 1 packet twice daily Methylprednisolone sodium succinate DEXAMETHASONE SODIUM CORTICOSTEROID 6/03 6/03 (Solu-Medrol®) injection PHOSPHATE injection If Solu-Medrol becomes unavailable due to Then interchange is approved for all shortage and the need to reserve a supply for indications except spinal cord injury and spinal cord injury… multiple sclerosis (Call physician to discuss alternatives to Solu-Medrol in MS.) (Adults) Total Daily Dose: (Adults) Convert to: <80 mg IV Dexamethasone at 1/5 the dose of Solu- Medrol given IV Q8H (i.e. Solu-Medrol 20 mg IV Q8H, change to Dexamethasone 4mg IV Q8H) 80-240 mg IV 8 mg IV Q8H 240-500 mg IV 10 mg IV Q8H >500 mg IV Call physician (Pediatrics) (Pediatrics) Convert to dexamethasone using Convert to dexamethasone using equipotent equipotent dosing (1:5). dosing (1:5). For example: Solu-Medrol 4mg Convert to: Dexamethasone 0.75mg Metronidazole IV (Adults) METRONIDAZOLE IV (Adults) ANTI-INFECTIVE 2/94 11/00 500mg IV Q6H 500mg IV Q8H (Peds) (Peds) 5-10mg/kg/dose IV Q 6H No interchange Micafungin (Mycamune®) injection CASPOFUNGIN (CANCIDAS®) ANTI-FUNGAL 6/06 6/06 INJECTION Invasive Candidiasis Micafungin 25-75mg IV daily(in clinical Caspofungin 70mg IV on day one, followed trials) by 50mg IV daily
Esophageal Candidiasis Micafungin 150mg IV daily Caspofungin 50mg IV daily
Empiric Prophylaxis of Neutropenic Fever Micafungin 50mg IV daily Caspofungin 70mg IV on day one, followed by 50mg IV daily
Invasive Aspergillosis Micafungin 75-150mg IV daily Caspofungin 70mg IV on day one, followed by 50mg IV daily
Moduretic (5/50) MAXZIDE (75/50) CARDIOVASCULAR 1/94 11/00 (11/93) Moexipril (Univasc®) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 2/97, 10/02 7.5mg PO DAILY 5mg PO DAILY 10/01 15mg PO DAILY 10mg PO DAILY
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Mometasone (Nasonex) Nasal Inhaler Fluticasone (Flonase) Nasal Inhaler INHALENT/NASAL 10/99 10/05 CORTICOSTEROIDS 2 sprays DAILY 2 sprays DAILY ADRENALS MOMETAXONE (ASMANEX® ***FORMULARY (Adults)*** INHALENT/NASAL 10/05 10/05 TWISTHALER) (Adults) CORTICOSTEROIDS ADRENALS (Peds) Fluticasone (Flonase®) (Peds) 1 puff daily 110mcg: puff twice daily 2 puffs daily 220mcg: 1 puff twice daily Monoket IMDUR CARDIOVASCULAR 11/95 11/00 10-20mg BID 30-60mg DAILY Moxifloxacin (Avelox) Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 6/00 3/06 500mg PO Q24H ABO 400mg PO Q24H Subcomm. Moxifloxacin (Avelox) IV LEVOFLOXACIN (Levaquin®) IV ANTI-INFECTIVE 6/00 3/06 500mg IV Q24H ABO 400mg IV Q24H Subcomm.
Moxifloxacin (Vigamox®) Ophthalmic ***FORMULARY*** ANTI-INFECTIVE 2/04 3/05 Reserved for cases of failed initial therapy, infection is severe, or risk factors that increase potential for more resistant or problematic organism – otherwise use ciprofloxacin or ofloxacin. Multivitamins Oral, (Adult) Generic Multivitamin, Oral (Adult), MISCELLANEOUS 11/93 9/04 tablet/capsule tablet Any Brand (i.e. Ocuvite, One-A-Day) Any schedule Same schedule
Multivitamin + Minerals Oral (Adult) Generic Multivitamin + Minerals, Oral MISCELLANEOUS 11/93 9/04 tablet/capsule (Adult), tablet Any Brand (i.e. Centrum, Centrum Silver) Any schedule Same schedule
Vitamin B complex with C, oral (Adult) Vitamin B complex with C, oral (Adult), MISCELLANEOUS 11/93 9/04 tablet/capsule tablet
Any brand (including Nephrocaps, Nephro-Vite RX, same schedule Renaphro, generic B+C), any schedule
Prenatal multivitamin, oral (Adult) Generic prenatal multivitamin, oral MISCELLANEOUS 11/93 9/04 (Adult) (i.e. Prenatal-Rx) Any brand, any schedule Same schedule Generic pediatric multivitamin, oral, MISCELLANEOUS 11/93 9/04 chewable (i.e. Fruity chews) Any brand, any schedule Same schedule Pediatric multivitamin with iron, oral, Generic pediatric multivitamin with iron, MISCELLANEOUS 11/93 9/04 chewable oral, chewable (i.e. Fruity chews with iron)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Same schedule ***FORMULARY*** MISCELLANEOUS 11/93 9/04 ***FORMULARY*** MISCELLANEOUS 11/93 9/04 Generic liquid multivitamin (Theravite), MISCELLANEOUS 11/93 9/04 oral, (Adult) Any brand, any dose, any schedule (i.e. 5ml PO once daily Theragran) Liquid multivitamin, oral (Peds) MISCELLANEOUS 11/93 9/04 Any brand (equivalent to Certagen), any Certagen, same dose (maximum 15 mL dose, any schedule daily), same schedule Generic multivitamin, oral drops (infant) MISCELLANEOUS 11/93 9/04 (i.e. Polyvitamin) Any brand (i.e. Poly-Vi-Sol, Vi-Daylin), Equivalent dose, same schedule any dose, any schedule Generic multivitamin, oral drops + iron MISCELLANEOUS 11/93 9/04 (infant) (i.e. Polyvitamin) Any brand (i.e. Poly-Vi-Sol, Vi-Daylin), Equivalent dose, same schedule any dose, any schedule Nabumetone (Relafen®) ***FORMULARY*** NSAIDS 5/05 5/05 Nafcillin IV (Adults) Oxacillin IV(Adults) ANTI-INFECTIVE 11/05 11/05 500mg IV any schedule 500mg IV same schedule 1000mg IV any schedule 1000mg IV same schedule 2000mg IV any schedule 2000mg IV same schedule (Pediatrics) (Pediatrics) 25mg/kg/dose, any schedule 25mg/kg/dose, same schedule 50mg/kg/dose for meningitis, any schedule 50mg/kg/dose for meningitis, same schedule Naproxen NA (Anaprox®) Oral NAPROXEN (Naprosyn®) Oral NSAIDS 1/94 5/05 275mg PO 250mg PO (11/93) Naratriptan (Amerge®) Oral SUMATRIPTAN (Imitrex®) Oral MISCELLANEOUS 10/01 10/03 1-2.5mg PO may repeat in 4 hrs 50mg PO may repeat in 2 hrs NTE 5mg/24 hrs NTE 200mg/24 hrs
Nasacort®, Nasacort AQ See Triamcinolone Nasal Inhaler Nasalide® See Flunisolide Nasal Inhaler Nasonex See Mometasone Nasal Inhaler Nebivolol (Bystolic®) Oral (Adults) CARVEDILOL (Coreg®) Oral (Adults) CARDIOVASCULAR 10/08 10/08 2.5-5mg PO daily 3.125 PO Twice Daily 10mg PO daily 6.25mg PO Twice Daily 20mg PO daily 12.5mg PO Twice Daily 30-40mg PO daily 25mg-50mg Twice Daily Nepafenac (Nevanac) Ophthalmic FLURBIPROFEN (OCUFEN) OPHTHALMIC 6/06 6/06 OPHTHALMIC 1 drop 3 times/day 1 drop 4 times/day
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Nicardipine (Cardene) Oral NIFEDIPINE XL (Procardia XL) CARDIOVASCULAR 3/94 11/00 30mg SR PO BID 30mg PO DAILY 45mg SR PO BID 30mg PO DAILY and titrate 60mg SR PO BID 60mg PO DAILY and titrate Nicotine Patch CURRENT BRAND NAME PRODUCT MISCELLANEOUS 1/94 11/00 ON BID FROM PURCHASING GROUP (11/93) Nifedipine Extended Release (Adalat Nifedipine Extended Release (Procardia CARDIOVASCULAR 1/03 1/03 CC®) Oral, Adults XL®) Oral, Adults 30 mg PO DAILY 30 mg PO DAILY 60 mg PO DAILY 60 mg PO DAILY 90mg PO DAILY 90 mg PO DAILY Nifedipine Extended Release (Procardia ***FORMULARY*** CARDIOVASCULAR 1/03 1/03 XL®) Nisoldipine (Sular®) AMLODIPINE (Norvasc®) CARDIOVASCULAR 10/96 11/00 10mg PO DAILY 2.5mg PO DAILY 20mg PO DAILY 5mg PO DAILY 30mg PO DAILY 7.5mg PO DAILY 40mg PO DAILY 10mg PO DAILY NIZATIDINE (Axid®) Oral (Adults, RANITIDINE (Zantac®) ORAL (Adults, GASTROINTESTINAL 1/01 1/01 Peds who can swallow capsule) Peds who can swallow capsule) 75 mg PO (Axid Acid Reducer®) 75 mg PO (Zantac 75®) 150mg PO QHS 150mg PO QHS 300mg PO QHS 300 mg PO QHS 150mg PO Q12H 150 mg PO Q12H Norfloxacin (Noroxin) Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 3/06 400mg PO Q12H 500mg PO Q24H (2/94) Ofloxacin (Floxin®) IV LEVOFLOXACIN (Levaquin®) IV ANTI-INFECTIVE 3/06 400mg IV Q12H 500mg IV Q24H 200mg IV Q12H 250mg IV Q24H Ofloxacin PO (Floxin®) Oral LEVOFLOXACIN (Levaquin®) Oral ANTI-INFECTIVE 3/06 400mg PO BID 500mg PO Q24H 200mg PO BID 250mg PO Q24H Ofloxacin Ophthalmic (Ocuflox®) ***FORMULARY*** ANTI-INFECTIVE 11/03 3/05 Olanzapine (Zyprexa®)injection ZIPRASIDONE (GEODON®) CENTRAL NERVOUS 2/06 2/06 injection SYSTEM AGENT 10mg 20mg Olmesartan (Benicar®) Oral VALSARTAN (DIOVAN®) Oral CARDIOVASCULAR 11/02 2/06 5mg PO DAILY 20mg PO DAILY 20mg PO DAILY 80mg PO DAILY 40mg PO DAILY 160mg PO DAILY Omeprazole (Prilosec) Oral (Adults) PANTOPRAZOLE (Protonix) Oral GASTROINTESTINAL 10/00 06/05 (Adults) 10mg – 20mg PO DAILY 40mg PO DAILY Ondansetron (Zofran®) IV (Peds) ***FORMULARY*** ANTI-EMETIC tentative 11/00 (Pediatric oral ondansetron dosing for (9/98) (2/98) gastroenteritis in ER (x1 dose only) <8 kg 0.2 mg/kg 8-15 kg 2mg
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 15-30 kg 4mg >30 kg 8mg Ondansetron (Zofran®) IV (Peds) ***FORMULARY*** ANTI-EMETIC tentative 11/00 Pediatric oral ondansetron for (9/98) (2/98) chemotherapy-induced N/V: Moderately emetogenic chemotherapy (category 2) (q 8 hours) <15 kg 0.15 mg/kg 15-30 kg 4mg >30 kg 8mg Orphenadrine Oral (Adults) CYCLOBENZAPRINE (FLEXERIL®) MUSCULOSKELETAL 06/05 06/05 100mg twice daily ORAL (Adults) 10mg three times daily > 65 years, 5mg three times daily Oxacillin Oral DICLOXACILLIN Oral ANTI-INFECTIVE (11/93) 11/00 500mg PO Q6H 125mg not available. Contact Prescriber. 1GM PO Q6H 250mg PO Q6H
Oxaprozin (Daypro®) NAPROXEN (Naprosyn®) Oral NSAIDS 5/05 5/05 600mg daily 250mg BID 1200mg daily 500mg BID Oxybutynin XL (Ditropan XL®) Oral SOLFENACIN (VESICARE®) ORAL MISCELLANEOUS 2/99 2/06 (Adults) (Adults) 5mg daily 5mg (1/2 x 10mg) daily 10mg daily 5mg (1/2 x 10mg) daily 15mg daily 10mg daily 20mg daily 10mg daily Oxycodone CR (Oxycontin®) Oral OXYCODONE/ACETAMINOPHEN ANALGESIC 10/01 10/01 For post-op pain (Percocet-5®) Oral (interchange for post-op pain--unless the patient has chronic pain and/or has been stabilized on Oxycontin due to intolerance of Oramorph®) 10mg Q12H 1 tablet Q6H 20mg Q12H 2 tablets Q6H (Use mg for mg equivalent daily dosing. If dosing at greater than 3 tablets Q6H, then switch to oxycodone plain due to acetaminophen component)
Oxymorphone (Opana®) or Morphine or Morphine Sulfate ER ANALGESIC 10/06 10/06 Oxymorphone ER (Opana®ER) (Dosage) (Opana Dosage x 3) Paliperidone (Invega®), Oral (Adults) Risperidone (Risperdal®), Oral (Adults) ANTIPSYCHOTIC 2/07 2/07 3 mg PO once daily 1 mg PO twice daily 6 mg PO once daily 3 mg PO twice daily 9 mg PO once daily 4 mg PO twice daily
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Palonosetron (Aloxi®) IV (Adults) Palonosetron is non- formulary, reserved ANTIEMETIC 09/05 4/07 0.25mg once for patients with prior history of acute or delayed Chemotherapy Induced Nausea and Vomiting not responsive to other anti- emetic regimens, and for patients with prior history or an expected occurrence of delayed Chemotherapy Induced Nausea and Vomiting not responsive to the current standard of care.
PANTOPRAZOLE (Protonix) (Adults) ***FORMULARY***(Adults) GASTROINTESTINAL 9/00 06/05 For feeding tube administration use (10/99) lansoprazole (Prevacid Solu-Tab) PANTOPRAZOLE (Protonix IV®) IV GASTROINTESTINAL 6/01 06/05 40mg (Adults) Patient with acute GI bleed or severe ***FORMULARY for these patients*** esophageal disorder intolerant to oral route 80mg IV Bolus followed by 8mg/hour for (i.e. NG suction, post-op ileus) 72 hours post intervention or 80mg IV Q8H 40 mg IV DAILY (double dose necessary for adequate treatment) for 72 hours post intervention. Patient without acute GI bleed or severe Interchange to Famotidine IV (Pepcid®) esophageal disorder, is not able to take oral (No advantage of PPI over H2 antagonist) tablets, does not have a feeding tube. Famotidine 20mg IV Q12H. 40 mg IV DAILY TPN patients: 40mg to TPN solution. Patient has a feeding tube Use lansoprazole (Prevacid Solu-Tab®) 40 mg IV DAILY (Adults) 30mg via NG tube DAILY Patient is able to take oral tablets Pantoprazole (Protonix®) Oral (Adults) 40 mg IV DAILY 40mg PO DAILY Paricalcitol (Zemplar) Oral, injection Doxercalciferol (Hectorol) Oral, ENDOCRINE 10/05 10/05 (Adults) injection (Adults) 1 mcg IV 0.5 mcg IV 1mcg PO 0.5 mcg PO Paroxetine Ext. Release (Paxil CR®) PAROXETINE (Paxil®) ANTIDEPRESSANT 10/02 10/02 (Adults) (Adults) 12.5 mg PO DAILY 10 mg PO DAILY 25 mg PO DAILY 20 mg PO DAILY 37.5 mg PO DAILY 30 mg PO DAILY Penbutolol (Levatrol) ATENOLOL CARDIOVASCULAR 4/94 11/00 20mg PO DAILY 50mg PO DAILY 40mg PO DAILY 100mg PO DAILY Penicillin G IV (During shortages only) Ampicillin IV ANTI-INFECTIVE 04/04 04/04 (adults) (adults) For GBS prophylaxis: For GBS prophylaxis: 5 million units IV for one dose, then 2.5 2 grams IV for one dose, then 1 gram IV million units IV every 4 hours until the every 4 hours until the patient delivers. patient delivers.
For other indications: For other indications:
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Any dose and schedule Call prescriber and discuss alternatives
(pediatrics) (pediatrics) Any indication, dose and schedule Call prescriber and discuss alternatives Percocet® Oral PERCOCET® (5mg/325mg Original ANALGESIC 2/00 6/07 strength) Oral No strength specified 5mg/325mg Percocet® 2.5mg/325mg 0.5 tablet of 5mg/325mg strength Percocet® 7.5mg/325mg 1.5 tablets of 5mg/325mg strength Percocet® 7.5mg/500mg 1.5 tablets of 5mg/325mg strength Percocet ® 10mg/325mg 2 tablets of 5mg/325mg strength Percocet® 10mg/650mg 2 tablets of 5mg/325mg strength PeriColace® (docusate/casanthranol) Docusate/Senna (Senokot-S) LAXATIVE 10/02 10/02 100mg/30mg 50mg/8.6mg 1 cap DAILY 1 tab PO BID 1 cap BID or 2 caps DAILY 2 tab PO BID 3 caps or greater daily dose 2 tab PO BID (maximum daily dose) Pediatrics: Pediatric dose: *for doses exceeding Docusate/Casanthranol (Peri-Colace) maximum recommended docusate range for (2/03) Capsules 100mg/30mg age call physician.
Syrup 60mg/30mg per 15ml SAME AS ABOVE
Change to Docusate Sodium Syrup (10mg/ml) mg for mg based on docusate component of Peri-Colace ordered AND Senna conc (Fletcher's Castoria 33.3mg/ml), dosing described below.
Infant –2 years old: 1.25 ml Senna conc QHS 3-6 years old: 2.5ml Senna conc QHS 6-12 years old: 5ml Senna conc QHS >12 years old: 10ml Senna conc QHS Perindopril (Aceon®) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 10/01 10/02 2mg PO DAILY 5mg PO DAILY 4mg PO DAILY 10mg PO DAILY 8mg PO DAILY 20mg PO DAILY Phenindamine (Nolahist®) Oral CYPROHEPTADINE Oral ANTIHISTAMINE 10/94 11/00 25mg PO Q4-6H 4mg PO Q8H
Phenylpropanolamine Oral PSEUDOEPHEDRINE Oral DECONGESTANT 10/94 11/00 25mg PO Q4H 60mg PO Q4-6H up to 240mg/DAY 50mg PO Q8H 75mg PO Q12H Phenylpropanolamine SR Oral PSEUDOEPHEDRINE Oral DECONGESTANT 10/94 11/00 125mg PO Q12H 60mg PO Q4-6H UP TO 240mg/DAY Phenylpropanolamine/ PSEUDOEPHEDRINE/ DECONGESTANT/ 11/00 11/00 Brompheniramine (Dimatapp®, BROMPHENIRAMINE (DIMETAPP®) ANTIHISTAMINE
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Genatap®) Elixir ELIXIR COMBINATION Equivalent dosing for Age Phenylpropanolamine/ PSEUDOEPHEDRINE/ DECONGESTANT/ 11/00 11/00 Chlorpheniramine/Phentoloxamine BROMPHENIRAMINE (DIMETAPP®) ANTIHISTAMINE COMBINATION (Naldecon®) Syrup ELIXIR Equivalent dosing for Age Phenytoin Injection – when IV access is FOSPHENYTOIN INJECTION MISCELLANEOUS 3/02 10/07 inappropriate per medication guideline (CEREBYX®) Interchange mg for mg Interchange mg for mg i.e. 100mg IV i.e. 100 mg PE IV Pindolol (Visken) ATENOLOL CARDIOVASCULAR 4/94 11/00 5mg PO DAILY BID 50mg PO DAILY 10mg PO DAILY BID 100mg PO DAILY Duetact® (pioglitazone/glimepiride) Pioglitazone (Actos®) + Glimepiride ANTIDIABETIC 10/06 10/06 Oral, Adults (Amaryl®) Oral, Adults 30mg/2mg once daily 30mg + 2mg once daily 30mg/4mg once daily 30mg + 4mg once daily Note: Duetact® should not be given more than once daily at any tablet strength as maximum recommended daily dose is 45mg/8mg. Actoplus met® (pioglitazone/metformin) Pioglitazone (Actos®) + metformin ANTIDIABETIC 10/06 10/06 Oral, Adults (Glucophage®) Oral, Adults 15mg/500mg once daily 15mg + 500mg once daily 15mg/500mg twice daily 15mg + 500mg twice daily 15g/850mg once daily 15mg + 850mg once daily 15mg/850mg twice daily 15mg + 850mg twice daily Three tablets of 15mg/850mg daily Three tablets 15mg + three tablets 850mg (45mg/2550mg total daily) daily with same schedule as combination Note: Maximum recommended daily dose product is 45mg/2550mg Pirbuterol (MAXAIR AUTOHALER®) ***FORMULARY*** INHALENT/NASAL 10/99 11/00 1-2 inhalations q4-6h. Only for use in CORTICOSTEROIDS SYMPATHOMIMETIC patients with difficulty coordinating (ADRENERGIC) albuterol MDI. Pirbuterol (Maxair®) ALBUTEROL (Proventil®/Ventolin®) INHALENT/NASAL 10/99 11/00 1-2 inhalations Q 4-6H 1-2 inhalations Q4-6H CORTICOSTEROIDS SYMPATHOMIMETIC (ADRENERGIC) Piroxicam (Feldene®) NAPROXEN (Naprosyn®) Oral NSAIDS 5/05 5/05 10mg daily 250 mg BID 20mg daily 500mg BID Pravastatin (Pravachol) SIMVASTATIN (Zocor®) CARDIOVASCULAR 10/96 11/00 10mg PO DAILY 5mg PO QHS (2/95) 20mg PO DAILY 10mg PO QHS 40mg PO DAILY 20mg PO QHS limit the simvastatin dose per day to 20mg when used concomitantly with amiodarone
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Procainamide SR (Procan SR®, PROCAINAMIDE SR (Procanbid®) CARDIOVASCULAR 2/00 11/00 Pronestyl-SR®) Oral Oral 500mg PO Q6H 1g PO Q12H 750mg PO Q6H 1.5g PO Q12H Any other dose Q6H or Q8H Give Procanbid® same total mg per 24H Split Q12H interchange approved for when procain-amide SR is not available
Proventil® See Albuterol Pulmicort Turbuhaler® See Budesonide Pyrilamine Oral CHLORPHENIRAMINE Oral ANTIHISTAMINE 10/94, 96 11/00 25-50mg PO Q6-8H 4mg PO Q4-6H
Quazepam (Doral®) Oral TEMAZEPAM (Restoril®) Oral SEDATIVE/ 1/94 11/00 7.5mg PO 15mg PO HYPNOTIC (11/93) Quetiapine XR (Seroquel XR®) Oral QUETIAPINE (Seroquel®) Oral (Adults) ANTIPSYCHOTIC 6/08 6/08 (Adults) divide the total daily dose into twice daily dosing of regular release quetiapine, for example: 200mg once daily 100mg twice daily 300mg once daily 150mg twice daily 400mg once daily 200mg twice daily Quinapril (Accupril) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 4/94, 10/02 5mg DAILY-BID 2.5mg-5mg PO DAILY 10/01 10mg DAILY-BID 5mg-10mg PO DAILY 20mg DAILY-BID 10mg-20mg PO DAILY 40mg DAILY 20mg PO DAILY
Rabeprazole (Aciphex) Oral (Adults) PANTOPRAZOLE (Protonix) Oral GASTROINTESTINAL 10/00 06/05 (Adults) 20mg-40mg PO DAILY 40mg PO DAILY Ramelteon (Rozerem®) ZOLPIDEM (AMBIEN®) SEDATIVE/ 6/06 6/06 8mg PO at bedtime 5 mg PO at bedtime HYPNOTIC Ramipril (Altace) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 4/94, 10/02 1.25mg DAILY-BID 2.5mg-5mg PO DAILY 10/01 2.5mg DAILY-BID 5mg-10mg PO DAILY 5mg DAILY-BID 10mg-20mg PO DAILY 10mg DAILY 20mg PO DAILY Ranitidine (Zantac®) IV (Adults) FAMOTIDINE IV (Adults) GASTROINTESTINAL 1/94 11/00 50mg IV Q8H 20mg IV Q12H (11/93) Ranitidine (Zantac®) Oral (Adults) ***FORMULARY*** GASTROINTESTINAL 1/01 (2/94), 1/01 Repaglinide (Prandin®), Oral NATEGLINIDE (STARLIX®), Oral ANTIDIABETIC 2/03 2/03 0.5- 2 mg PO TID 120 mg PO TID Repaglinide/Metformin (PrandiMet®) Repaglinide + METFORMIN ANTIDIABETIC 10/08 10/08 1mg/500mg any schedule 1mg + 500mg same schedule, same total
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed daily dose 2mg/500mg any schedule 2mg + 500mg same schedule, same total daily dose Note: Maximum recommended total daily dose is 10mg/2500mg; no more than 4mg/100mg should be taken per meal. Retapamulin (Altabax®) 1% Ointment Mupirocin (Bactroban®) 2% Ointment ANTI-INFECTIVE 2/08 2/08 Applied twice daily Applied three times daily Rhinocort® See Budesonide Nasal Inhaler Rizatriptan (Maxalt®) Oral SUMATRIPTAN (Imitrex®) Oral MISCELLANEOUS 10/01 10/03 5-10mg PO may repeat in 2 hrs 50mg PO may repeat in 2 hrs NTE 30mg/24 hrs NTE 200mg/24 hrs Ropinirole Extended Release (Requip Ropinirole Immediate Release, Oral MISCELLANEOUS 10/08 10/08 XL™) Oral Adults (Adults) (Total daily dose divided three times daily) 2 mg PO DAILY 1.5 mg (0.5 mg three times daily) 4 mg PO DAILY 3.75 mg (1.25 mg three times daily) 6 mg PO DAILY 6 mg (2 mg three times daily) 8 mg PO DAILY 7.5 mg (2.5 mg three times daily) 12 mg PO DAILY 12 mg (4 mg three times daily) 16 mg PO DAILY 18 mg (6 mg three times daily) 20 mg PO DAILY 21 mg (7 mg three times daily) 24 mg PO DAILY 24 mg (8 mg three times daily) Rosuvastatin (Crestor®) Oral (Adults) SIMVASTATIN (ZOCOR ®) Oral CARDIOVASCULAR 10/03 10/03 (Adults) 5mg PO once daily 20mg PO once daily 10mg PO once daily 40mg PO once daily 20mg or 40mg PO once daily 80 mg PO once daily Note: an increase in myalgia and limit the simvastatin dose per day to rhabdomyolysis was seen at doses greater 20mg when used concomitantly with than 40mg in clinical trials. The FDA has amiodarone limited maximum dosing to 40mg daily. Saccharomyces boulardii (Florastor®) Culturelle GG (Lactobacillus casei, MISCELLANEOUS 10/08 10/08 subspecies rhamnosus GG) Oral, 10 billion CF units/capsule. (Adults and Pediatrics) 1 capsule PO BID 1 capsule PO Daily Salmeterol (Serevent®) Diskhaler Formoterol (Foradil®) Aerolizer ORAL 10/99 6/03 (Adults) (Adults) CORTICOSTEROIDS SYMPATHOMIMETIC 1 inhalation (50mcg) Q12H 1 inhalation (12mcg) Q12H (ADRENERGIC) (Pediatrics) (Pediatrics) See adult dose See adult dose for children > 5 years SALSALATE (Disalcid®) ** FORMULARY** NSAIDS 5/05 5/05 Sargramostim (Leukine®) FILGRASTIM (Neupogen®) MISCELLANEOUS 5/08 5/08 Dose: 250 mcg/m2 subcutaneously daily Dose: 5 mcg/kg Sub-Q daily. If BSA ≤ 1.3 m2 give 250 mcg/dose If < 80kg, give 300mcg dose If BSA >1.3 m2 and ≤ 2.3 m2 give 500 If ≥ 80kg, give 480mcg dose mcg/dose If BSA > 2.3 m2 give 750 mcg/dose
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed Sevelamer Carbonate (Renvela®) 800mg SEVELAMER HYDROCHLORIDE MISCELLANEOUS 6/08 6/08 (Renagel®) 800mg 1 tab three times daily with meals 1 tab three times daily with meals 2 tab three times daily with meals 2 tab three times daily with meals Sildenafil (Revatio™) Oral (Adults) SILDENAFIL (VIAGRA®) Oral MISCELLANEOUS 10/05 10/05 (Adults) 20mg any schedule for Pulmonary Arterial 25mg (1/2 of 50mg) same schedule for Hypertension Pulmonary Arterial Hypertension Simvastatin (Zocor) ***FORMULARY*** CARDIOVASCULAR 10/96 11/00 limit the simvastatin dose per day to 20mg when used concomitantly with amiodarone Stalevo(Levodopa/Carbidopa/ CARBIDOPA/LEVODOPA MISCELLANEOUS 10/04 10/04 Entacapone) Oral (Adults) ½ tablet of regular release 25 mg/100 mg 12.5 mg-50-200 mg Plus ENTACAPONE 200 mg
25 mg-100-200 mg CARBIDOPA/LEVODOPA 1 tablet of regular release 25 mg/100 mg Plus ENTACAPONE 200 mg
37.5 mg-150-200 mg CARBIDOPA/LEVODOPA 1 ½ tablet of regular release 25 mg/100 mg Plus ENTACAPONE 200 mg Sulindac (Clinoril®) ***FORMULARY*** NSAIDS 5/05 5/05 Sumatriptan (Imitrex®) Oral ***FORMULARY*** MISCELLANEOUS 10/01 2/03
Sumatriptan 85mg/Naproxen 500mg Sumatriptan (Imitrex®) + Naproxen MISCELLANEOUS 10/08 10/08 (Treximet ™ ) Oral (Adults) (Naprosyn®) Oral (Adults) 85 mg/500 mg 50 mg + 500 mg Sutilains (Travase®) UREA+PAPAIN (Accuzyme®) Unless for ENZYMATIC 2/98 11/00 debridement of cancer patients with necrotic DEBRIDING AGENTS tissue Symbyax ® (olanzapine/fluoxetine) Oral Olanzapine (Zyprexa®) and Fluoxetine CENTRAL NERVOUS 02/04 02/04 (Adults) (Prozac®) Oral (Adults) SYSTEM AGENT Any frequency Same frequency 6mg/25mg Olanzapine 5mg + fluoxetine 20mg 6mg/50mg Olanzapine 5mg + fluoxetine 40mg 12mg/25mg Olanzapine 10mg + fluoxetine 20mg 12mg/50mg Olanzapine 10mg + fluoxetine 40mg Telmisartan (Micardis®) Oral VALSARTAN (DIOVAN®) Oral CARDIOVASCULAR 2/99 2/06 20mg PO DAILY 40mg PO DAILY 40mg PO DAILY 80mg PO DAILY 80mg PO DAILY 160mg PO DAILY TEMAZEPAM ***FORMULARY*** SEDATIVE/ 6/06 6/06 HYPNOTIC Terazosin (Hytrin®) Oral DOXAZOSIN (Cardura®) Oral CARDIOVASCULAR 5/98 11/00
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 1mg PO DAILY 1 mg PO DAILY (4/94) Tdap(tetanus, diphtheria, and pertussis) Tdap (tetanus, diphtheria, and pertussis) VACCINE 10/05 10/05 (Boostrix®) IM (10-18 years old) (AdacelTM) IM (11-64 years old) 0.5mL IM injection 0.5mL IM injection Thiazides Oral (Adults) HYDROCHLOROTHIAZIDE (Adults) CARDIOVASCULAR 1/94 11/00 (Equivalent Dose) Timentin® (Ticarcillin/clavulanic acid) ZOSYN® (PIPERACILLIN/ ANTI-INFECTIVE 6/01 6/01 IV TAZOBACATAM) IV (Peds, except Cystic Fibrosis) (Peds) 200-300 mg/kg/day 240-400 mg/kg/day divided Q8H (Adults) (Adults) 2 gm IV Q8H 3.375 gm IV Q12H 3.1 gm IV Q12H 3.375 gm IV Q12H 3.1 gm IV Q8H 3.375 gm IV Q8H 3.1 gm IV Q6H 4.5 gm IV Q8H 3.1 gm IV Q4H 4.5 gm IV Q8H Timolol (Blocadren) Oral ATENOLOL Oral CARDIOVASCULAR 4/94 11/00 10mg PO BID 50mg PO DAILY 20mg PO BID 100mg PO DAILY Tinzaparin (Innohep®) Injection DALTEPARIN(Fragmin®) INJECTION CARDIOVASCULAR 3/01 5/01
General Surgery VTE prophylaxis General Surgery VTE prophylaxis 3,500 units SC DAILY Moderate Risk: 2,500 units SC DAILY
Orthopedic Surgery VTE prophylaxis Orthopedic Surgery VTE prophylaxis 75 units/Kg SC DAILY starting 12-24h 2,500 units SC 6 hours postop x1, then postop or 4,500 units SC DAILY 5,000 units SC DAILY (if LMWH to start postop day 1, then start 5000 units SC DAILY)
Treatment DVT/PE Treatment DVT/PE 175 units/Kg SC DAILY 200 units/kg SC DAILY Maximum daily dose: 18,000 units
BID dosing (dalteparin 100 units/kg SC BID) may be considered for high-risk patients (ie malignancy, ileofemoral thrombosis, recurrent DVT/PE). Tizanidine tablets/capsules (Zanaflex®) Cyclobenzaprine (Flexeril®) Oral MUSCULOSKELETAL 06/05 06/05 Oral (Adults) (Adults) 4mg three times daily 8mg three times daily 5 mg three times daily 10mg three times daily > 65 years, 5mg three times daily Tolmetin (Tolectin/DS®) IBUPROFEN Oral NSAIDS 5/05 5/05 400mg Q8H 400mg Q6H 600mg Q8H 600mg Q6H Tolterodine (Detrol) Oral (Adults) SOLFENACIN (VESICARE®) Oral MISCELLANEOUS 10/03 6/06 (Adults)
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 1mg BID 5mg (1/2 x 10mg) daily 2mg BID 10mg daily
Tolterodine LA (Detrol LA) Oral SOLFENACIN (VESICARE®) Oral MISCELLANEOUS 10/03 6/06 (Adults) (Adults) 2mg daily 5mg (1/2 x 10mg) daily 4mg daily 10mg daily Tornalate® See Bitolterol Trandolapril (Mavik®) Oral LISINOPRIL (Zestril®) Oral (Adults) CARDIOVASCULAR 10/96, 10/02 1mg PO DAILY 5mg PO DAILY 10/01 2mg PO DAILY 10mg PO DAILY 4mg PO DAILY 20mg PO DAILY Travase® See Sutilains ENZYMATIC DEBRIDING AGENT Triamcinolone (Nasacort®, Nasarel) FLUTICASONE (Flonase) Nasal In- INHALENT/NASAL 10/99 11/00 CORTICOSTEROIDS Nasal Inhaler (Adults) haler (Adults) ADRENALS 2 sprays DAILY 2 sprays DAILY 3 sprays DAILY 4 sprays DAILY Triamcinolone 100mcg (Azmacort®) FLUTICASONE 44mcg (Flovent®) INHALENT/NASAL 2/00 10/02 (Peds) (Peds) CORTICOSTEROIDS (10/99) 2-4 puffs BID 2 puffs BID 1 puff TID-QID 2 puffs BID 2 puffs TID-QID 2 puffs BID (Adults) (Adults) FLUTICASONE 44mcg (Flovent®) 1puff QID 1 puff BID 2 puffs QID 2 puffs BID 3 puffs QID 3 puffs BID 4 puffs QID 4 puffs BID Triamterene (Dyrenium®) Oral (Adults) AMILORIDE (Midamor®) Oral (Adult) CARDIOUVASCULAR 9/01 9/01 50 mg any schedule 5 mg same schedule Triazolam/Halcion Oral (Adult) TEMAZEPAM (RESTORIL) Oral SEDATIVE/ 1/94 6/06 (Adult) HYPNOTIC (11/93) 0.125 mg PO at bedtime 15mg PO at bedtime 0.25 mg PO at bedtime 30mg PO at bedtime Except for sleep clinic, pre-op use, pregnant and breast feeding mothers, and pediatric Triazolam/Halcion patients; then: 0.125 mg PO at bedtime ZOLPIDEM (AMBIEN®) 0.25 mg PO at bedtime 5 mg PO at bedtime 10 mg PO at bedtime
Tripelennamine (PBZ®) Oral CHLORPHENIRAMINE Oral ANTIHISTAMINE 10/94 11/00 25-50mg PO Q6-8H 4mg PO Q4-6H Tripolidine (Actidil®) Oral CHLORPHENIRAMINE Oral ANTIHISTAMINE 10/94 11/00 2.5mg PO Q4-6H 4mg PO Q4-6H Trospium (Sanctura®) Oral (Adults) SOLFENACIN (VESICARE®) Oral MISCELLANEOUS 2/06 2/06
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed (Adults) 20mg daily 5mg (1/2 x 10mg) daily 20mg BID 10mg daily
(Truvada™ ) Emtricitabine/Tenofovir EMTRICITABINE (EMTRIVA®) Oral ANTIVIRAL 10/04 10/04 Oral (Adults) (Adults) 200mg/300mg PO once daily 200mg PO once daily Plus TENOFOVIR (VIREAD®) Oral (Adults) 300mg PO once daily Ultracet® (tramadol 37.5mg/ Tramadol 50mg (Ultram) tablet ANALGESIC 6/02 6/02 acetaminophen 325mg) Oral and Acetaminophen 325mg tablet Valacyclovir (Valtrex®) Oral ACYCLOVIR (Zovirax®) Oral ANTI-VIRAL 11/96 6/02 (Adults) 1 gm PO Q8H or Q12H 800mg 5 times daily (CrCl >=25 ml/min)
1gm PO, other schedule: Acyclovir dosed appropriately for renal function as follows: 800mg PO Q8H (CrCl 10-25 ml/min) 800mg PO Q12H (CrCl <10 ml/min)
1 gm PO Q12H or 500mg PO Q12H 200mg PO 5 times daily (CrCl >10ml/min) (for initial treatment or recurrence) 200mg PO Q12H (CrCl<10 ml/min)
1 gm PO DAILY (for suppression) 400mg PO Q12H (CrCl >10 ml/min) 200mg PO Q12H (CrCl < 10 ml/min)
Oral, Pediatrics: Call prescriber and recommend acyclovir dosing based on appropriate indication Valdecoxib (Bextra®) Oral (Adults) CELECOXIB (CELEBREX®) Oral NSAIDS 2/02 10/04 (Adults) 10mg PO once daily 200mg PO once daily 20mg PO once daily 200mg PO twice daily 20mg PO twice daily 200mg PO twice daily Valsartan (Diovan®) Oral ***FORMULARY*** CARDIOVASCULAR 2/99 2/06 40mg PO DAILY (10/97) (6/97) 80mg PO DAILY or 40mg PO BID 160mg PO DAILY or 80mg PO BID 320mg PO DAILY or 160mg PO BID Ventolin® See Albuterol Hydrocodone 7.5 mg + Ibuprofen 200mg HYDROCODONE 7.5 MG + APAP 500 ANALGESIC 2/03 2/03 (Vicoprofen®) Oral mg (Lortab®) Oral 1 tablet 1 tablet Vivelle® Estrogen Patch See Estrogen Patch Vivelle-DOT® Estrogen Patch See Estrogen Patch Vytorin™ (Ezetimibe/Simvastatin) Oral EZETIMIBE (Zetia®) + SIMVASTATIN CARDIOVASCULAR 10/04 10/04 (Adults) (Zocor®) Oral (Adults) 10mg/10mg PO daily 10mg + 10mg PO daily
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Note: Orally disintegrating tablet formulations may be interchanged to standard oral tablet or capsule at equivalent doses (and vice versa) based on availability and cost, except in cases where the patient has difficulty swallowing a standard oral tablet or capsule. Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed 10mg/20mg PO daily 10mg + 20mg PO daily 10mg/40mg PO daily 10mg + 40mg PO daily 10mg/80mg PO daily 10mg + 80mg PO daily limit the simvastatin dose per day to limit the simvastatin dose per day to 20mg when used concomitantly with 20mg when used concomitantly with amiodarone amiodarone Zafirlukast (Accolate®) MONTELUKAST (SINGULAIR®) Oral RESPIRATORY 5/98 11/00 20mg PO BID 10mg PO DAILY Zaleplon/Sonata ZOLPIDEM (AMBIEN®) SEDATIVE/ 10/99 6/06 5mg PO at bedtime 5 mg PO at bedtime HYPNOTIC 10 mg PO at bedtime 10 mg PO at bedtime Zileuton (Zyflo®) Oral MONTELUKAST (Singulair®) Oral RESPIRATORY 6/98 11/00 600mg PO QID 10mg PO DAILY (adults) 5mg PO DAILY (peds) Zoledronic acid (Zometa®) injection PAMIDRONATE (AREDIA®) injection MISCELLANEOUS 1/05 1/05 (adults) (adults) 4 mg IV over 15 minutes 90 mg IV over 2 hours For CrCl 45-60 ml/min run over 4 hours For CrCl 30-44 ml/min run over 6 hours For CrCl <30 ml/min run over 8 hours Zolmitriptan (Zomig®) Oral SUMATRIPTAN (Imitrex®) ORAL MISCELLANEOUS 10/01 10/03 ***Interchange unless has failed Sumatriptan already, then is formulary*** 50mg PO may repeat in 2 hrs 2.5mg PO may repeat in 2 hrs NTE 200mg/24 hrs NTE 10mg/24 hrs ZOLPIDEM (AMBIEN®) ***FORMULARY*** SEDATIVE/ 6/06 6/06 HYPNOTIC Zolpidem CR (Ambien CR) ZOLPIDEM (AMBIEN®) SEDATIVE/ 2/06 6/06 6.25 mg PO at bedtime 5 mg PO at bedtime HYPNOTIC 12.5 mg PO at bedtime 10 mg PO at bedtime ZOSYN® (PIPERACILLIN/ ***FORMULARY*** ANTI-INFECTIVE 6/01 6/01 TAZOBACATAM) IV
TOPICAL ANTIFUNGALS Drug/Dose Therapeutic Interchange Medication Class Date Date approve reviewed d Ciclopirox (Loprox®) 0.77% Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 cream/gel/susp, 8% solution 1% Cream/solution Apply twice daily Apply twice daily Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 Cream/solution **FORMULARY AGENT**
Econazole (Spectazole®) 1% cream Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 1% Cream/solution Apply daily to twice daily Apply twice daily Ketoconazole (Nizoral®, Xolegel®, Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 Extina®) 2% Cream 1% Cream/solution
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Drug/Dose Therapeutic Interchange Medication Class Date Date approve reviewed d Apply daily to twice daily Apply twice daily Miconazole (micatin®, fungoid®) 2% Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 cream 1% Cream/solution Apply twice daily Apply twice daily Miconazole (Mitrazol®) 2% Powder ANTI-INFECTIVE 2/08 2/08 **FORMULARY AGENT** Nystatin (Nilstat®,Mycostatin®) ANTI-INFECTIVE 2/08 2/08 100,000units/g cream/ointment **FORMULARY AGENT** Nystatin (Nystop®, Mycostatin®) Miconazole (Mitrazol®) 2% Powder ANTI-INFECTIVE 2/08 2/08 100,000 unit/g powder Apply daily to twice daily Apply daily to twice daily Oxiconazole (Oxistat®) 1% Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 cream/lotion 1% Cream/solution Apply daily to twice daily Apply twice daily Sulconazole (Exelderm®) 1% Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 cream/solution 1% Cream/solution Apply twice daily Apply twice daily Terbinafine (Lamisil AT®) 1% cream Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 1% Cream/solution Apply daily to twice daily Apply twice daily Tolnaftate (Tinactin®) 1% Clotrimazole (Lotrimin®, Mycelex®) ANTI-INFECTIVE 2/08 2/08 cream/powder/soln 1% Cream/solution Apply twice daily Apply twice daily
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST TOPICAL CORTICOSTEROIDS Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed augmented betamethasone dipropionate CLOBETASOL PROPIONATE VERY HIGH 6/94 5/05 cream 0.05% (Diprolene) CREAM 0.05% (Temovate) POTENCY TOPICAL CORTICOSTEROID augmented betamethasone dipropionate CLOBETASONL PROPIONATE VERY HIGH 6/94 5/05 ointment 0.05% (Diprolene) OINTMENT 0.05% (Temovate) POTENCY TOPICAL CORTICOSTEROID clobetasol propionate cream 0.05% ***FORMULARY*** VERY HIGH 6/94 5/05 (Temovate) POTENCY TOPICAL CORTICOSTEROID clobetasol propionate ointment 0.05% ***FORMULARY*** VERY HIGH 6/94 5/05 (Temovate) POTENCY TOPICAL CORTICOSTEROID halobetasol propionate cream 0.05% CLOBETASOL PROPIONATE VERY HIGH 6/94 5/05 (Ultravate) CREAM 0.05% (Temovate) POTENCY TOPICAL CORTICOSTEROID halobetasol propionate ointment 0.05% CLOBETASOL PROPIONATE VERY HIGH 6/94 5/05 (Ultravate) OINTMENT 0.05% (Temovate) POTENCY TOPICAL CORTICOSTEROID amcinonide ointment 0.1% BETAMETHASONE HIGH POTENCY 6/94 5/05 (Cyclocort) DIPORPIONATE OINTMENT TOPICAL 0.05% (Diprosone) CORTICOSTEROID betamethasone dipropionate ointment ***FORMULARY*** HIGH POTENCY 6/94 5/05 0.05% (Diprosone) TOPICAL CORTICOSTEROID desoximetasone cream 0.25% ***FORMULARY*** HIGH POTENCY 6/94 5/05 (Topicort) TOPICAL CORTICOSTEROID desoximetasone ointment 0.25% BETAMETHASONE HIGH POTENCY 6/94 least 5/05 (Topicort) DIPROPIONATE OINTMENT TOPICAL irritating 0.05% (Diprosone) CORTICOSTEROID when applied to eczematou s skin diflorasone diacetate ointment 0.05% BETAMETHASONE HIGH POTENCY 6/94 5/05 (Florone) DIPROPIONATE OINTMENT TOPICAL 0.05% (Diprosone) CORTICOSTEROID fluocinonide cream 0.05% (Lidex) ***FORMULARY*** HIGH POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID fluocinonide ointment 0.05% (Lidex) BETAMETHASONE HIGH POTENCY 6/94 5/05 DIPROPIONATE OINTMENT TOPICAL 0.05% (Diprosone) CORTICOSTEROID fluocinonide solution 0.05% (Lidex) ***FORMULARY*** HIGH POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID halcinonide cream 0.1% (Halog) FLUOCINONIDE CREAM 0.05% HIGH POTENCY 6/94 5/05 (Lidex) TOPICAL CORTICOSTEROID amcinonide cream 0.1% (Cyclocort) BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 CREAM 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed betamethasone benzoate cream 0.025% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Uticort) CREAM 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID betamethasone dipropionate cream BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 0.05% (Diprosone) CREAM 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID betamethasone dipropionate lotion BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 0.05% (Diprosone) LOTION 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID betamethasone valerate cream 0.1% ***FORMULARY*** INTERMEDIATE 6/94 5/05 (Valisone) POTENCY TOPICAL CORTICOSTEROID betamethasone valerate lotion 0.1% ***FORMULARY*** INTERMEDIATE 6/94 5/05 (Valisone) POTENCY TOPICAL CORTICOSTEROID betamethasone valerate ointment 0.1% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Valisone) OINTMENT 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID
FLUOCINOLONE ACETONIDE OINTMENT 0.025% (Synalar) (cost-effective when larger amounts required) diflorasone diacetate cream 0.05% BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 (Florone) CREAM 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID fluocinolone acetonide cream 0.01% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Synalar) CREAM 0.1% (Aristocort) POTENCY TOPICAL CORTICOSTEROID fluocinolone acetonide cream 0.025% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Synalar) CREAM 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID fluocinolone acetonide cream 0.2% BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 (Synalar-HP) CREAM 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID fluocinolone acetonide ointment ***FORMULARY*** INTERMEDIATE 6/94 5/05 0.025% (Synalar)(cost effective when POTENCY TOPICAL larger amounts are required) CORTICOSTEROID flurandrenolide cream 0.05% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Cordran) CREAM 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID flurandrenolide ointment 0.05% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Cordran) OINTMENT 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID
FLUOCINOLONE ACETONIDE OINTMENT 0.025% (Synalar) (cost-effective when larger amounts required) hydrocortisone butyrate cream 0.1% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Locoid) CREAM 0.1% POTENCY TOPICAL
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed (Kenalog/Aristocort) CORTICOSTEROID hydrocortisone valerate cream 0.2% TRIAMCINOLONE ACETONIDE INTERMEDIATE 6/94 5/05 (Westcort) CREAM 0.1% POTENCY TOPICAL (Kenalog/Aristocort) CORTICOSTEROID Mometasone Fumarate (Elocon®) TRIAMCINOLONE ACETONIDE INTERMEDIATE 5/05 5/05 Ointment 0.1% Ointment 0.1% POTENCY TOPICAL (Kenalog/Aristocort®) CORTICOSTEROID Mometasone Fumarate (Elocon®) BETAMETHSONE VALERATE INTERMEDIATE 5/05 5/05 Cream 0.1% Cream 0.1% (Valisone®) POTENCY TOPICAL CORTICOSTEROID triamcinolone acetonide 0.1% lotion BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 (Kenalog) LOTION 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID Triamcinolone acetonide cream 0.1% ***FORMULARY*** INTERMEDIATE 6/94 5/05 (Kenalog/Aristocort) INTERMEDIATE POTENCY TOPICAL CORTICOSTEROID triamcinolone acetonide cream 0.5% BETAMETHASONE VALERATE INTERMEDIATE 6/94 5/05 (Kenalog) CREAM 0.1% (Valisone) POTENCY TOPICAL CORTICOSTEROID triamcinolone acetonide ointment ***FORMULARY*** INTERMEDIATE 6/94 5/05 0.1% (Kenalog/Aristocort) POTENCY TOPICAL CORTICOSTEROID alclometasone dipropionate cream HYDROCORTISONE CREAM 1% LOW POTENCY 6/94 5/05 0.05% (Aclovate) TOPICAL CORTICOSTEROID alclometasone dipropionate ointment HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 0.05% (Aclovate) 1% TOPICAL CORTICOSTEROID clocortolone pivalate cream 0.1% TRIAMCINOLONE ACETONIDE LOW POTENCY 6/94 5/05 (Cloderm) CREAM 0.1% (Aristocort) TOPICAL CORTICOSTEROID desonide cream 0.05% (Tridesilon) HYDROCORTISONE CREAM 1% LOW POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID desonide ointment 0.05% HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 (Tridesilon) 1% TOPICAL CORTICOSTEROID dexamethasone cream 0.1% (Decadron HYDROCORTISONE CREAM 1% LOW POTENCY 6/94 5/05 Phosphate) TOPICAL CORTICOSTEROID flurandrenolide cream 0.025% TRIAMCINOLONE ACETONIDE LOW POTENCY 6/94 5/05 (Cordran) CREAM 0.1% (Aristocort) TOPICAL CORTICOSTEROID flurandrenolide ointment 0.025% TRIAMCINOLONE ACETONIDE LOW POTENCY 6/94 5/05 (Cordran) CREAM 0.1% (Aristocort) TOPICAL CORTICOSTEROID hydrocortisone cream 0.5% HYDROCORTISONE CREAM 1% LOW POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID hydrocortisone cream 1% ***FORMULARY*** LOW POTENCY 6/94 5/05 TOPICAL
Revised: October 2008 THERAPEUTIC INTERCHANGE PROGRAM MHS ACUTE CARE Pharmacy and Therapeutics Committee APPROVED LIST Drug/Dose Therapeutic Interchange Medication Class Date Date approved reviewed CORTICOSTEROID hydrocortisone cream 2.5% HYDROCORTISONE CREAM 1% LOW POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID hydrocortisone ointment 0.5% HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 1% TOPICAL CORTICOSTEROID hydrocortisone ointment 1% ***FORMULARY*** LOW POTENCY 6/94 5/05 TOPICAL CORTICOSTEROID hydrocortisone ointment 2.5% HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 1% TOPICAL CORTICOSTEROID methylprednisolone ointment 0.25% HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 1% TOPICAL CORTICOSTEROID methylprednisolone ointment 1% HYDROCORTISONE OINTMENT LOW POTENCY 6/94 5/05 1% TOPICAL CORTICOSTEROID
Revised: October 2008