Chemistry 122- Organic Chemistry Unit Section 22.4, Page 709-711
Objective: Identify and name cyclic ring structures. Describe the bonding in Benzene. Name aromatic compounds.
HYDROCARBON RINGS
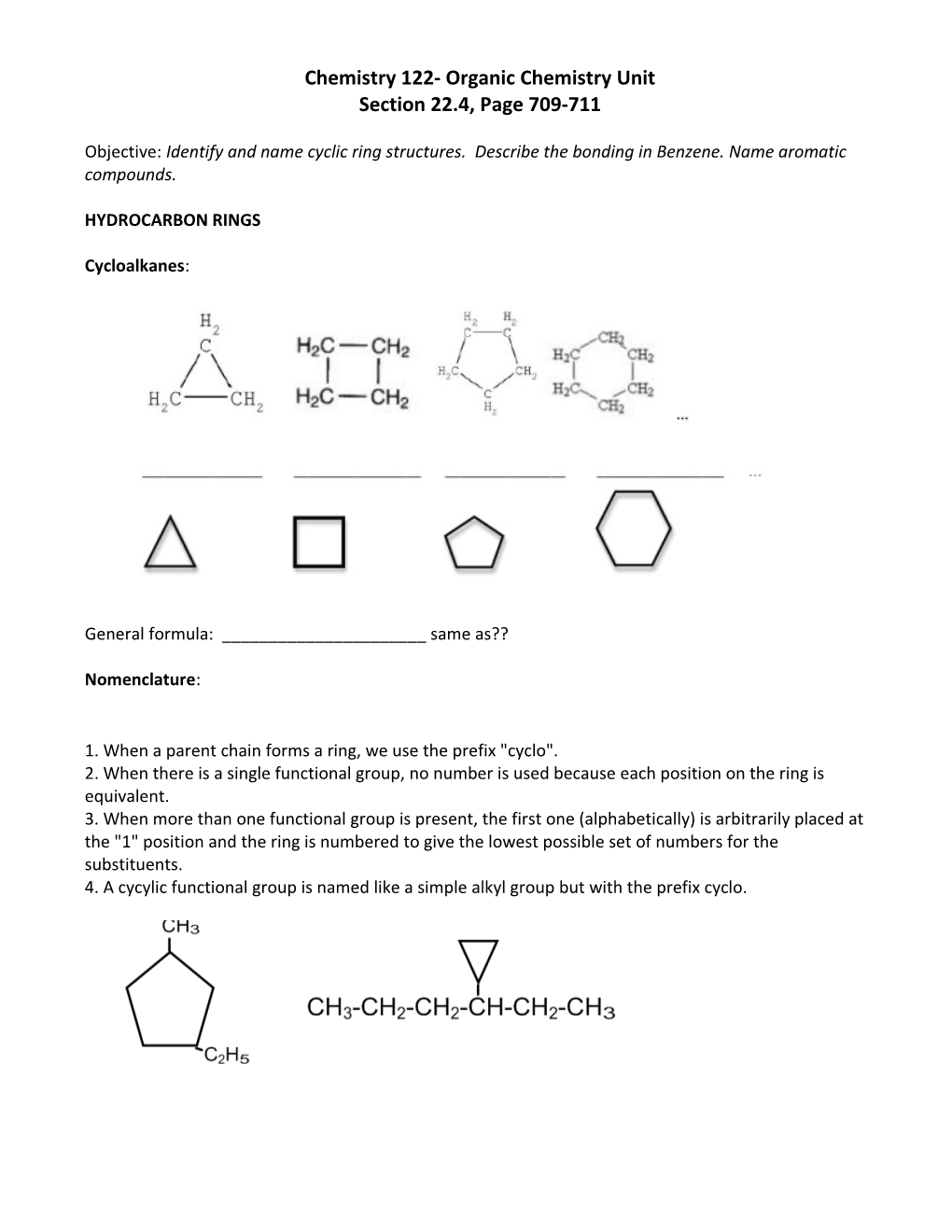
Cycloalkanes:
General formula: ______same as??
Nomenclature:
1. When a parent chain forms a ring, we use the prefix "cyclo". 2. When there is a single functional group, no number is used because each position on the ring is equivalent. 3. When more than one functional group is present, the first one (alphabetically) is arbitrarily placed at the "1" position and the ring is numbered to give the lowest possible set of numbers for the substituents. 4. A cycylic functional group is named like a simple alkyl group but with the prefix cyclo. Aromatics (arenes): These compounds usually have a pleasant aroma. Some examples include wintergreen, aspirin, vanilla, cloves, cinnamon, ginger, benzocaine. This class of aromatic organic compounds share a common structure, the ______ring, ______
BENZENE: -colorless, flammable liquid -Pleasant sweet smell - Carcinogenic -found in gasoline, napalm, carcinogenic -found in gasoline, napalm, crude oil -important ______solvent: if it doesn't dissolve in ______, it will probably dissolve in benzene.
Structure:
-flat or ______-non-polar -all C-C bonds the same length (140pm) between a double (134pm) and single bond (146pm) bond length- Hybrid -exhibits ______-very ______
Nomenclature: Name the substituent followed by benzene is only one functional group.
1. Number the carbon atoms to give them the lowest numbers possible 2. Determine the name of the substituents, use di, tri.. etc if more than of the same is present. 3. Put the name together and alphabetize the substituent names, and use commas between numbers and hyphens between numbers and words. 4. When there are two functional groups in the 1,2 position it is known as ______(o-), the 1,3 position is ______(m-) and the 1,4 position is ______(p-). 5. If there is a large group attached to the benzene ring, then the benzene becomes the functional group. A benzene group is called "______".