Supplementary Information – Namiki et al. - 1
SUPPLEMENTARY INFORMATION – FIGURES and TABLES
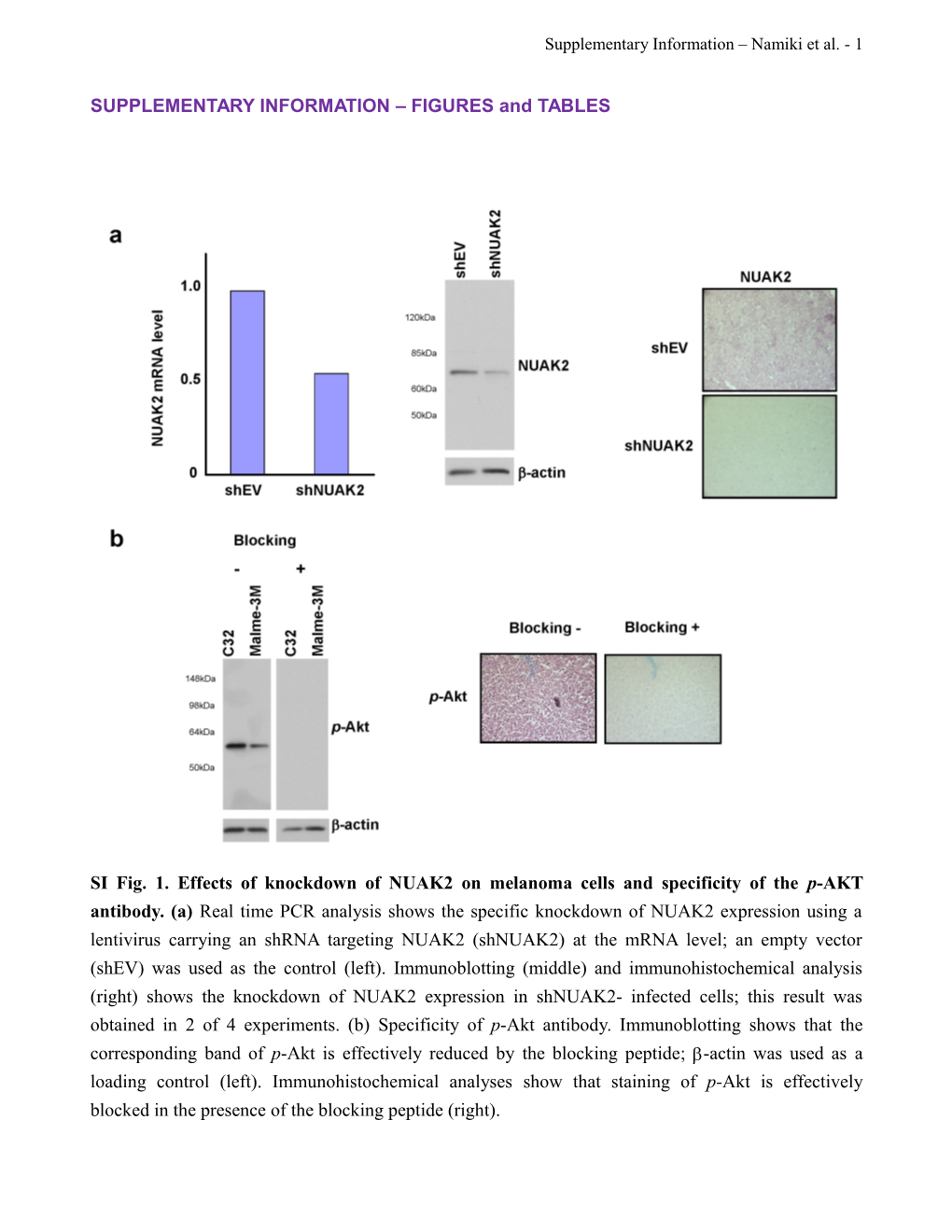
SI Fig. 1. Effects of knockdown of NUAK2 on melanoma cells and specificity of the p-AKT antibody. (a) Real time PCR analysis shows the specific knockdown of NUAK2 expression using a lentivirus carrying an shRNA targeting NUAK2 (shNUAK2) at the mRNA level; an empty vector (shEV) was used as the control (left). Immunoblotting (middle) and immunohistochemical analysis (right) shows the knockdown of NUAK2 expression in shNUAK2- infected cells; this result was obtained in 2 of 4 experiments. (b) Specificity of p-Akt antibody. Immunoblotting shows that the corresponding band of p-Akt is effectively reduced by the blocking peptide; -actin was used as a loading control (left). Immunohistochemical analyses show that staining of p-Akt is effectively blocked in the presence of the blocking peptide (right). Supplementary Information – Namiki et al. - 2
SI Fig. 2. Immunohistochemical analyses of p-Akt and NUAK2 expression in clinical specimens. p-Akt and NUAK2 were highly-expressed in the acral melanoma of case No.23 and that staining was c ounted as strong (+3) (upper panel). p-Akt and NUAK2 were not expressed in the acral melanoma of c ase No.24 and that staining was counted as negative (0) (middle panel). p-Akt and NUAK2 were high or not expressed, respectively, in the non-CSD melanoma of case No.59 and those staining were counte d as (+3) and (0), respectively (lower panel). Supplementary Information – Namiki et al. - 3
a AcralAcral melanoma melanoma 0 . 1 e 8 . g 0 a t n e c r 6 e . P = 0.072 p 0
l a v i v r 4 . u s 0
l l a r e 2 v . O 0 Neither NUAK2 nor p-AKT positive Either NUAK2 or p-AKT positive 0
. Both NUAK2 and p-AKT positive 0
1 2 3 4 5 6 7 8 9 10 11 12 13
time(year) b Non-CSDNon-csd melanoma melanoma 0 . 1 P = 0.669 e 8 . g a 0 t n e c r 6 e . p 0
l a v i v r 4 . u s 0
l l a r e 2 v . O 0 Neither NUAK2 nor p-AKT positive Either NUAK2 or p-AKT positive 0
. Both NUAK2 and p-AKT positive 0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
time(year) SI Fig. 3. Kaplan-Meier curves for overall survival of Acral and Non-CSD melanoma patients. (a) The overall survival time of acral melanoma patients with expression of both NUAK2 and p- Akt(S473) was shorter than the survival time of acral melanoma patients with either NUAK2 or p- Akt(S473) alone (P = 0.072). (b) The overall survival time of Non-CSD melanoma patients with expression of both NUAK2 and p-Akt(S473) was not different from those of either NUAK2 or p- Akt(S473) alone (P = 0.669). A log rank test was used to detect survival time differences among 3 groups of patients: 1) Neither NUAK2 nor p-Akt(S473) positive; 2) Either NUAK2 or p-Akt(S473) positive; 3) Both NUAK2 and p-Akt(S473) positive. If we only compare groups 3 and 2 in acral melanomas, the p value is 0.074. Supplementary Information – Namiki et al. - 4
SI Fig. 4. Knockdown of NUAK2 induces apoptosis in SM2-1 melanoma cells. Cells were stained with Annexin V-APC and 7-ADD, followed by analyses on a flow cytometer. Knockdown of NUAK2 by shNUAK2 dramatically induced apoptosis in SM2-1 melanoma cells (3.35 to 12.02) compared to C 32 melanoma cells (10.69 to 28.03). Supplementary Information – Namiki et al. - 5
SI Fig. 5. Immunohistochemistry showing the expression of p21 following inhibition of the PI3K pathway by LY294002. Cell numbers expressing p21 are significantly increased (P < 0.001) following inhibition of the PI3K pathway. Supplementary Information – Namiki et al. - 6
SI Fig. 6. Schematic diagram of the regulation of the cell cycle machinery by NUAK2 and PI3K p athways. Predicted activated pathways in both NUAK2 amplified and PTEN deleted melanoma cells ar e depicted as red arrows. The cell cycle machinery in both NUAK2 amplified and PTEN deleted melan oma cells is speculated to be controlled through inhibition of both p21CIP1 and G0 transition. Predicte d pathways are shown with dotted lines. Supplementary Information – Namiki et al. - 7
SI Fig. 7. Knockdown of CDK2 by siCDK2 in C32 and mel18 melanoma cells. (a) CDK2 expressio n is suppressed by siCDK2 at 24 h, 48 h and 72 h in C32 melanoma cells. -actin was used as a loadin g control. (b) CDK2 expression is suppressed by siCDK2 at 24 h, 48 h and 72 h in mel18 melanoma ce lls. -actin was used as a loading control. Supplementary Information – Namiki et al. - 8
SI Fig. 8. Immunohistochemical analyses of the expression of CDK2 in clinical specimens. NUAK 2, CDK2 expression and H&E staining of case No. 26 are shown. NUAK2 and CDK2 are highly-expre ssed. Supplementary Information – Namiki et al. - 9
SI Fig. 9. Expression of NUAK2, PTEN, p-Akt, Akt and CDK2, and cell proliferation in various melanoma cell lines treated with Roscovitine. (a) Immunoblotting showing expression of NUAK2, P TEN, p-Akt, Akt and CDK2 in C32, mel2, mel18, A375, Malme-3M, SKMel28 and SKMel23 melano ma cells. -actin was used as a loading control. (b) Cell proliferation assays of mel2, A375, SKMel28 a nd SKMel23 melanoma cells treated with Roscovitine at 0, 5 and 25 M. Supplementary Information – Namiki et al. - 10
SI Table 1. Clinical parameters and expressions of CDK2, p-Akt and NUAK2 of 56 acral melanomas and 35 non-CSD melanomas with survival information. Supplementary Information – Namiki et al. - 11
SI Table 2. Genomic status of NUAK2 and PTEN gene in each cell line. Genomic status of NUAK2 and PTE N are shown by DNA copy number and deletion, respectively. SM2-1, mel18 and mel2 are acral melanoma cell l ines. SKMel28 has a mutation of A499G at PTEN gene (1). Supplementary Information – Namiki et al. - 12
SI Table 3. Expression of p27 and CDK2 in primary melanomas with or without over-expression of NUAK2 and p-Akt Supplementary Information – Namiki et al. - 13
Reference
1) Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res 2006;12;2301s-2307s.