ANNEX.
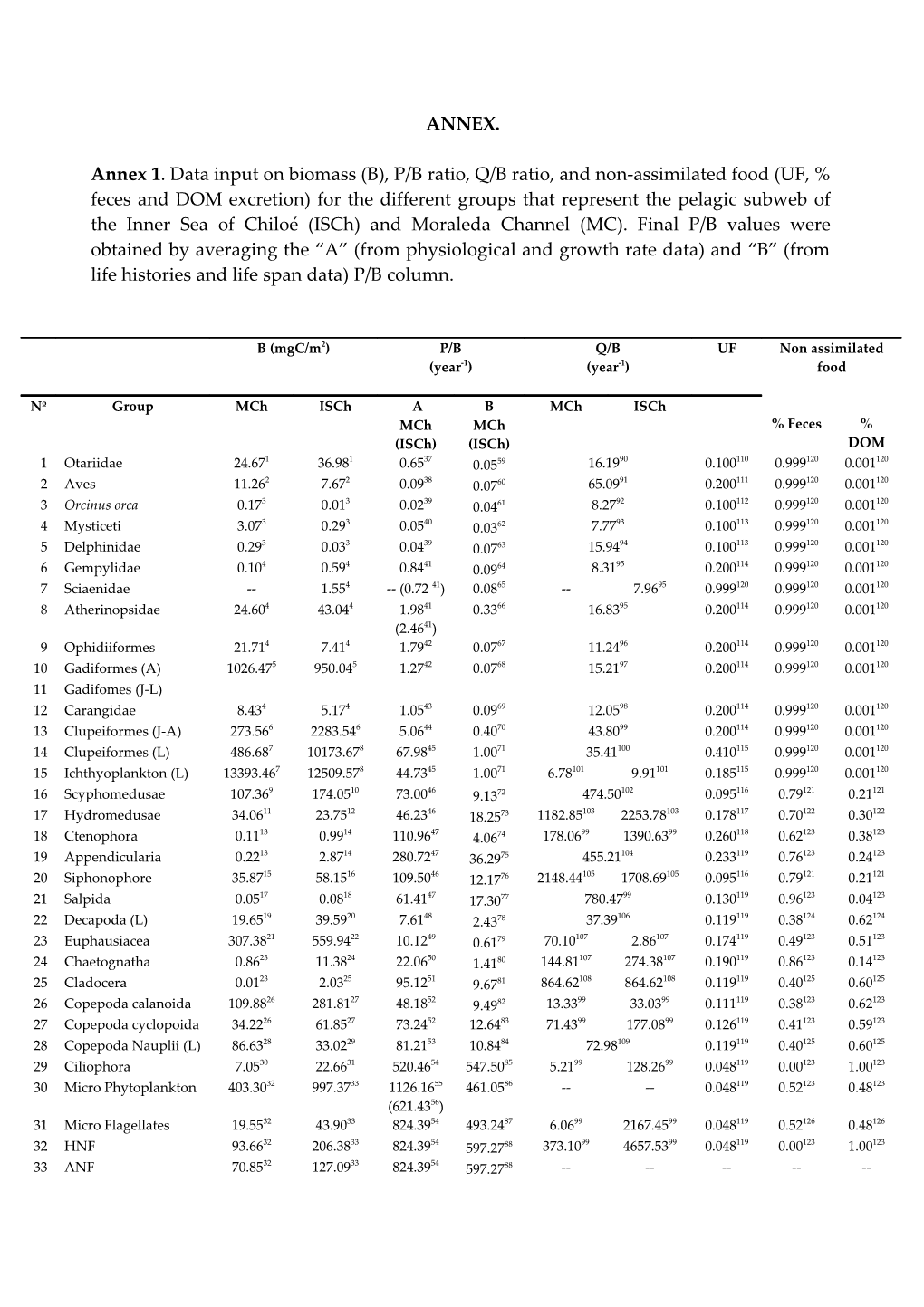
Annex 1. Data input on biomass (B), P/B ratio, Q/B ratio, and non-assimilated food (UF, % feces and DOM excretion) for the different groups that represent the pelagic subweb of the Inner Sea of Chiloé (ISCh) and Moraleda Channel (MC). Final P/B values were obtained by averaging the “A” (from physiological and growth rate data) and “B” (from life histories and life span data) P/B column.
B (mgC/m2) P/B Q/B UF Non assimilated (year-1) (year-1) food
Nº Group MCh ISCh A B MCh ISCh MCh MCh % Feces % (ISCh) (ISCh) DOM 1 Otariidae 24.671 36.981 0.6537 0.0559 16.1990 0.100110 0.999120 0.001120 2 Aves 11.262 7.672 0.0938 0.0760 65.0991 0.200111 0.999120 0.001120 3 Orcinus orca 0.173 0.013 0.0239 0.0461 8.2792 0.100112 0.999120 0.001120 4 Mysticeti 3.073 0.293 0.0540 0.0362 7.7793 0.100113 0.999120 0.001120 5 Delphinidae 0.293 0.033 0.0439 0.0763 15.9494 0.100113 0.999120 0.001120 6 Gempylidae 0.104 0.594 0.8441 0.0964 8.3195 0.200114 0.999120 0.001120 7 Sciaenidae -- 1.554 -- (0.72 41) 0.0865 -- 7.9695 0.999120 0.999120 0.001120 8 Atherinopsidae 24.604 43.044 1.9841 0.3366 16.8395 0.200114 0.999120 0.001120 (2.4641) 9 Ophidiiformes 21.714 7.414 1.7942 0.0767 11.2496 0.200114 0.999120 0.001120 10 Gadiformes (A) 1026.475 950.045 1.2742 0.0768 15.2197 0.200114 0.999120 0.001120 11 Gadifomes (J-L) 12 Carangidae 8.434 5.174 1.0543 0.0969 12.0598 0.200114 0.999120 0.001120 13 Clupeiformes (J-A) 273.566 2283.546 5.0644 0.4070 43.8099 0.200114 0.999120 0.001120 14 Clupeiformes (L) 486.687 10173.678 67.9845 1.0071 35.41100 0.410115 0.999120 0.001120 15 Ichthyoplankton (L) 13393.467 12509.578 44.7345 1.0071 6.78101 9.91101 0.185115 0.999120 0.001120 16 Scyphomedusae 107.369 174.0510 73.0046 9.1372 474.50102 0.095116 0.79121 0.21121 17 Hydromedusae 34.0611 23.7512 46.2346 18.2573 1182.85103 2253.78103 0.178117 0.70122 0.30122 18 Ctenophora 0.1113 0.9914 110.9647 4.0674 178.0699 1390.6399 0.260118 0.62123 0.38123 19 Appendicularia 0.2213 2.8714 280.7247 36.2975 455.21104 0.233119 0.76123 0.24123 20 Siphonophore 35.8715 58.1516 109.5046 12.1776 2148.44105 1708.69105 0.095116 0.79121 0.21121 21 Salpida 0.0517 0.0818 61.4147 17.3077 780.4799 0.130119 0.96123 0.04123 22 Decapoda (L) 19.6519 39.5920 7.6148 2.4378 37.39106 0.119119 0.38124 0.62124 23 Euphausiacea 307.3821 559.9422 10.1249 0.6179 70.10107 2.86107 0.174119 0.49123 0.51123 24 Chaetognatha 0.8623 11.3824 22.0650 1.4180 144.81107 274.38107 0.190119 0.86123 0.14123 25 Cladocera 0.0123 2.0325 95.1251 9.6781 864.62108 864.62108 0.119119 0.40125 0.60125 26 Copepoda calanoida 109.8826 281.8127 48.1852 9.4982 13.3399 33.0399 0.111119 0.38123 0.62123 27 Copepoda cyclopoida 34.2226 61.8527 73.2452 12.6483 71.4399 177.0899 0.126119 0.41123 0.59123 28 Copepoda Nauplii (L) 86.6328 33.0229 81.2153 10.8484 72.98109 0.119119 0.40125 0.60125 29 Ciliophora 7.0530 22.6631 520.4654 547.5085 5.2199 128.2699 0.048119 0.00123 1.00123 30 Micro Phytoplankton 403.3032 997.3733 1126.1655 461.0586 -- -- 0.048119 0.52123 0.48123 (621.4356) 31 Micro Flagellates 19.5532 43.9033 824.3954 493.2487 6.0699 2167.4599 0.048119 0.52126 0.48126 32 HNF 93.6632 206.3833 824.3954 597.2788 373.1099 4657.5399 0.048119 0.00123 1.00123 33 ANF 70.8532 127.0933 824.3954 597.2788 ------34 Bacteria 225.5032 453.3233 34.2257 1460.008 1268.8899 861.3699 0.050119 0.00123 1.00123 (129.1458) 9
35 DOM 24330.4834 34503.4534 ------1.00123 36 Detritus 4055.0835 5750.5836 ------1.00123 --
A) Biomass: 1 = 6 2 6 2 BOtariidae = ∑Bsex /Area (AreaMch = 8,263.0x10 m ; or, AreaISCh = 9,675.0x10 m ); Where, Bsex = SP * Ab * Ww * CCF / Area; Where, SP = Sex proportion (Oporto et al., 1999); Ab = Abundance (Oporto et al., 1999); Ww = wet weight (Palomares & Pauly, 2011); CCF = Carbon Conversion Factor, 1g ww = 0.114 gC indiv -1
(Cauffopé & Heymans, 2005); Area = Area (AreaMch or AreaISCh); 2 = BAves = Ab * Ww * CCF / Area; Where, Ab = Abundance (Hucke-Gaete et al., 2010); Ww = wet weight (Palomares & Pauly, 2011); CCF = Carbón Conversion Factor, 1g ww = 0.114 gC indiv -1 (Cauffopé &
Heymans, 2005); Area = Area (AreaMch or AreaISCh); 3 = Bi = Ab * Ww * CCF / Area; Where, Ab = Abundance (Aguayo et al., 2006; Hucke-Gaete et al., 2010; Viddi et al., 2010; Zamorano-Abramson et al., 2010), Ww = wet weight (Kenney et al., 1997; Palomares & Daniel Pauly, 2011), CCF = Carbon Conversion Factor, 1g ww = 0.114 gC indiv -1 (Cauffopé & Heymans,
2005), Area = Area (AreaMch or AreaISCh); 4 = Bi = (Ti * CCF ) / Area; Where, Ti = ton specie “i”; CCF = Carbon Conversion Factor, 0.06 gC indiv -1
(Walsh, 1981); Area = Area (AreaMch or AreaISCh); Ti = (Bclup * %Li) / %Lclup; Where, Ti = ton specie “i”; Bclup = Clupeiformes Biomass; %Li = Percentage of specie “i” in the landing for MCh or ISCh (SERNAPESCA, 2003; SERNAPESCA, 2006; SERNAPESCA, 2007; SERNAPESCA, 2008; SERNAPESCA, 2009); %Lclup = Percentage of Clupeiformes species in the landing for MCh or ISCh (SERNAPESCA, 2003; SERNAPESCA, 2006; SERNAPESCA, 2007; SERNAPESCA, 2008; SERNAPESCA, 2009); 5 = Bi = ( SEi * CFC ) / Area; Where, For Gadiformes Biomass, SEi = biomass from stock assessments (Lillo et al., 2004, et al., 2006, et al., 2008); CCF = Carbón Conversion Factor, 0.06 gC indiv -1 (Walsh, 1981); Area
(AreaMch or AreaISCh); For Gadiformes (J-L) were estimated from EwE; 6 = SEi = biomass from stock assessments (Niklitschek et al., 2009); CCF = Carbon Conversion Factor, 0.06 -1 gC indiv (Walsh, 1981); Area (AreaMch or AreaISCh); 7 = Biomass = larval number m-3 (Landaeta & Castro, 2006) * 50m * larval wet weight (Bustos et al., 2008, Niklitschek et al., 2009) * 0.06 gC indiv -1 (Walsh, 1981); 8 = Biomass = larval number m-3 (Bustos et al., 2008) * 50m * larval wet weight (Bustos et al., 2008; Niklitschek et al., 2009) * 0.06 gC indiv -1 (Walsh, 1981); 9 = Estimated from relationship BScypho/BSiphono obtained for ISCh; 10 = Biomass = N° indiv. m-3 (Palma et al., 2011) * 50m * 1265.8 mgC indiv -1 (Shenker, 1985); 11 = Biomass = N° indiv. m-3 (Palma et al., 2007) * 50m * 165.60 µgrC indiv -1 (assumed); 12 = Biomass = N° indiv. m-3 (Villenas et al., 2009; Palma et al., 2011) * 50m * 165.60 µgrC indiv -1 (assumed); 13 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13; Ctenophora = 128.4 µgC ind-1 (Hirst et al., 2003); Appendicularia = 4.39 µgC ind-1 (Hirst et al., 2003); 14 = Abundance m2 from unpublished zooplankton data from 9 stations and 2 depths during CIMAR 12; Ctenophora = 128.4 µgC ind-1 (Hirst et al., 2003); Appendicularia = 4.39 µgC ind-1 (Hirst et al., 2003); 15 = Biomass = N° indiv. m-3 (Palma et al., 2007) * 50m * 180.30 µgrC indiv -1 (Purcell & Kremer, 1983); 16 = Biomass =N° indiv. m-3 (Villenas et al., 2009; Palma et al., 2011) *50m * 180.30 µgrC indiv -1 (Purcell & Kremer, 1983); 17 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13; Salpida = 188.47 µgC ind-1 (González et al., 2000); 18 = Abundance m2 from unpublished zooplankton data from 9 stations and 2 depths during CIMAR 12; Salpida = 188.47 µgC ind-1 (González et al., 2000); 19 = Biomass = N° indiv. m-3 (Mujica, 2008) * 50m * 0.25 annually (Pérez-Barros et al., 2007) * 159.25 µgrC indiv -1 (Uye, 1982); 20 = Biomass = N° indiv. m-3 (Mujica & Nava, 2010; Mujica et al., 2011) * 50m * 0.33 annually (Pérez-Barros et al., 2007) * 159.25 µgrC indiv -1 (Uye, 1982); 21 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13 (González et al., 2011); Euphausiacea = 649-5353 µgC ind-1 (González et al., 2000; Hirst et al., 2003; González et al., 2011); 22 = Abundance m2 from unpublished zooplankton data from 9 stations and 2 depths during CIMAR 12 (H. E. González et al., 2010); Euphausiacea = 649-5353 µgC ind-1 (González et al., 2000; Hirst et al., 2003, González et al., 2011); 23 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13; Chaetognatha = 107.69 µgC ind-1 (Pearre, 1992; González et al., 2000; Hirst et al., 2003); Cladocera =1.93 µgC ind-1 (Sánchez et al., 2011); 24 = Biomass = N° indiv. m-3 (Villenas et al., 2009) * 50m * 107.69 µgC ind-1(Pearre, 1992; González et al., 2000; Hirst et al., 2003); 25 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13; Cladocera =1.93 µgC ind-1 (Sánchez et al., 2011); 26 = Abundance m2 from unpublished zooplankton data from 13 stations and 2 depths during CIMAR 13; calanoida = 91.04 µgC ind-1; cyclopoida = 6.37 µgC ind-1 (González et al., 2000; González et al., 2010; González et al., 2011); 27 = Abundance m2 from unpublished zooplankton data from 11 stations and 2 depths during CIMAR 12; calanoida = 91.04 µgC ind-1; cyclopoida = 6.37 µgC ind-1 (González et al., 2000, et al., 2010, et al., 2011); 28 = Nauplii Biomass (González et al., 2011), where 11 samples stations were considered in the present study; 29 = Nauplii Biomass (González et al., 2010), where 9 samples stations were considered in the present study; 30 = Biomass = biomass (González et al., 2011) * 1.56 CF (Stoecker et al., 1994), where 26 samples stations were considered in the present study); 31 = Biomass = biomass obtained (González et al., 2010) * 1.56 CF (Stoecker et al., 1994), where 26 samples stations were considered in the present study; 32 = Biomass (González et al., 2011), where 11 samples stations were considered in the present study; 33 = Biomass (González et al., 2010), where 13 samples stations were considered in the present study; 34 = Biomass DOM = Detritus * 6 (Pavés et al. submitted); 35 = POC – Biomass functional Groups 29-34 (González et al., 2011), where 11 samples stations were considered in the present study; 36 = POC – Biomass functional Groups 29-34 (González et al., 2010), where 13 samples stations were considered in the present study.
B) Production/Biomass ration (P/Bi) P/B in days * 365, or, in year; 37 = P/Bi = P/B mean obtained from other models (Neira & Arancibia, 2004; Neira et al., 2004); 38 = P/B mean obtained from other models (Sidi & Guénette, 2004; Medina et al., 2007; Melgo et al., 2009; Morissette et al., 2010); 39 = P/B mean obtained from other models (Sidi & Guénette, 2004; Melgo et al., 2009; Morissette et al., 2010); 40 = P/B mean obtained from other models (Melgo et al., 2009; Morissette et al., 2010); 41 = P/B mean obtained from other models (Sidi & Guénette, 2004; Melgo et al., 2009); 42 = P/B mean obtained from other models (Neira et al., 2004; Sidi & Guénette, 2004; Morissette et al., 2010); 43 = P/B mean obtained from other models (Neira et al., 2004; Sidi & Guénette, 2004; Medina et al., 2007; Melgo et al., 2009; Morissette et al., 2010); 44 = P/B obtained from relationship P/B = Z (Cubillos et al., 2007); 45 = P/B from growth rates (Houde, 1989); 46 = P/B from growth rates (Larson, 1986); 47 = P/B from growth rates (Hirst et al., 2003; Sato et al., 2008); 48 = P/B from P/B Euphausiacea (Annex 1) and P/B Amphipoda (Ikeda & Shiga, 1999); 49 = P/B from growth rates (Hirst et al., 2003; Pinchuk & Hopcroft, 2006; Shaw et al., 2010); 50 = P/B from growth rates (Newbury, 1978; Hirst et al., 2003); 51 = P/B from growth rates, P = daily growth rate (Preuss et al., 2009), B = Bi (Annex 1). 52 = P/B from growth rates (Hirst et al., 2003); 53 = P/B from growth rates (Uye & Sano, 1998; Hirst et al., 2003); 54 = P/B obtained from other model (Pavés & González, 2008); 55 = P/B from PP value (PP = 1244 mg C m-2 d-1; González et al., 2011) and relationship with Micro Phytoplankton Biomass 56 = P/B from PP value (PP = 1698 mg C m-2 d-1; González et al., 2010) and relationship with Micro Phytoplankton Biomass 57 = P/B from PBS value (González et al., 2011) and relationship with Bacteria Biomass 58 = P/B from PBS value (González et al., 2010) and relationship with Bacteria Biomass B) Production/Biomass ration, P/Bi = 1/MLS; MLS = mean life span; P/Best in days * 365, or, in year; 59 = MLS = mean life span, 20 years (Sielfeld et al., 1997; Palomares & Pauly, 2011); 60 = MLS = 15 years (Palomares & Pauly, 2011); 61 = MLS = 25 years (Palomares & Pauly, 2011); 62 = MLS = 40 years (Palomares & Pauly, 2011); 63 = MLS = 15 years (Palomares & Pauly, 2011); 64 = MLS = 11.5 years (Horn, 2002; Acuña et al., 2007); 65 = MLS = 13 years (Oyarzún et al., 1999); 66 = MLS = 3 years (Pavez et al., 2008); 67 = MLS = 14.5 years (Withell & Wankowski, 1989; Wiff et al., 2007); 68 = MLS = 15 years (Aguayo & Ojeda, 1987; Lillo et al., 2005; Chong et al., 2007; Aguayo et al., 2010; Cerna, 2003); 69 = MLS = 11 years (Cubillos & Arancibia, 1995; Karlou-Riga & Sinis, 1997; Córdova et al., 2006); 70 = MLS = 2.5 years (Gru & Cousseau, 1982; Canales & Leal, 2009; Niklitschek et al., 2009; Castillo-Jordán et al., 2010); 71 = MLS = 1 year (assumed); 72 = MLS = 40 days (assumed); 73 = MLS = 20 days (assumed); 74 = MLS = 90 days (Hirota, 1974); 75 = MLS = 10.6 days (López-Urrutia et al., 2003; Deibel & Lowen, 2011); 76 = MLS = 30 days (Carre & Carre, 1991); 77 = MLS = 21.1 days (Deibel & Lowen, 2011); 78 = MLS = 150 days (assumed); 79 = MLS = 1.6 years (Ross, 1982; Taki, 2004; Hamame & Antezana, 2010); 80 = MLS = 258.5 days (Giesecke & González, 2008); 81 = MLS = 37.7 days (Bottrell, 1975; Lynch, 1980); 82 = MLS = 38.5 days (Huntley & Lopez, 1992); 83 = MLS = 28.9 days (Huntley & Lopez, 1992); 84 = MLS = 33.7 days (assumed); 85 = MLS = 0.7 days (Dolana & Coats, 1990; Strom & Morello, 1998); 86 = MLS = 0.8 days (Redalje & Laws,1981); 87 = MLS = 0.7 days (Anderson, 1998; Strom & Morello, 1998); 88 = MLS = 0.6 days (Dolana & Coats, 1990; Drebes et al., 1996); 89 = MLS = 0.3 days (assumed); C) Comsuption/Biomass ration. 90 = Q/B from ingestion rate and Q/B mean from others models (George-Nascimento et al., 1985; Kastelein et al., 1995; Neira et al., 2004) in relation to its own biomass; 91 = Q/B from ingestion rate and Q/B mean from other models (Santos et al., 2001; Sidi & Guénette, 2004; Medina et al., 2007; Melgo et al., 2009; Morissette et al., 2010) in relation to its own biomass; 92 = Q/B mean from other models (Sidi & Guénette, 2004; Melgo et al., 2009; Morissette et al., 2010) in relation to its own biomass; 93 = Q/B mean from other models (Santos et al., 2001; Morissette et al., 2009, et al., 2010) in relation to its own biomass; 94 = Q/B from ingestion rate and Q/B mean from other models (Cheal & Gales, 1991; Sidi & Guénette, 2004; Melgo et al., 2009; Morissette et al., 2010) in relation to its own biomass; 95 = Q/B mean from other models (Sidi & Guénette, 2004; Melgo et al., 2009); 96 = Q/B from ingestion rate and Q/B mean from other models (Macpherson, 1983; Sidi & Guénette, 2004; Neira et al., 2004; Morissette et al., 2010) in relation to its own biomass; 97 = Q/B from ingestion rates and Q/B mean from other models (Prenski & Angelescu, 1993; Neira et al., 2004; Sidi & Guénette, 2004; Morissette et al., 2009; Morissette et al., 2010; Durbin et al., 1983) in relation to its own biomass; 98 = Q/B mean from other models (Sidi & Guénette, 2004; Medina et al., 2007; Melgo et al., 2009; Morissette et al., 2010) in relation to its own biomass; 99 = Q/B from other model (Pavés & González, 2008) in relation to its own biomass; 100 = Q/B from ingestion rates (McGurk, 1984; Angelescu & Anganuzzi, 1986) in relation to its own biomass; 101 = Q/B from ingestion rates (Walline, 1987) in relation to its own biomass; 102 = Q/B from ingestion rates (Suchman et al., 2008) in relation to its own biomass; 103 = Q/B from ingestion rates (Marshalonis & Pinckney, 2008) in relation to its own biomass; 104 = Q/B from ingestion rates (Scheinberg et al., 2005) in relation to its own biomass; 105 = Q/B from ingestion rates (Purcell & Kremer, 1983) in relation to its own biomass; 106 = Q/B averaged from Q/B of functional groups 23, 26, 28; 107 = Q/B from ingestion rates (Pavés & González, 2008) in relation to its own biomass; (copepods, 48.7 µgC indiv -1); 108 = Q/B from ingestion rates (Sánchez et al., 2011) in relation to its own biomass; 109 = Q/B from ingestion rates (Meyer et al., 2002; Irigoien et al., 2003) in relation to its own biomass. D) Non assimilated food 110 = Rosen et al. (2000); 111 = Gabrielsen & Brekke (1994); 112 = Williams et al. (2004); 113 = Hain et al. (1985); 114 = Christensen & Walter (2000); 115 = Govoni et al. (1986); 116 = Purcell (1983); 117 = UF averaged from UF functional groups 16 and 18; 118 = Reeve et al. (1978); 119 = Pavés & González (2008). E) %DOM and POC 120 = Assumed; 121 = Mean %DOM and POC from groups number 18 and 21; 122 = Mean %DOM and POC from groups number 16 and 18; 123 = From (Pavés & González, 2008); 124 = Assumed from functional groups, 26; 125 = Mean %DOM and POC from groups number 26 and 27; 126 = Assumed from functional groups, 30. Annex 2. Diet composition of the pelagic subweb of the Inner Sea of Chiloé (regular) and Moraleda Channel (in bold). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 1 Otariidae 0.040 2 Aves 0.011 3 Orcinus orca 4 Mysticeti 0.010 5 Delphinidae 0.184 (0.183) 6 Gempylidae 0.032 0.026 0.001 0.033 (0.033) (0.025) (0.036) 7 Sciaenidae 0.324 0.063 0.030 0.067 0.015 (0.000) (0.000) (0.000) (0.000) (0.000) 8 Atherinopsidae 0.132 0.011 0.004 0.011 0.088 0.015 0.088 0.011 (0.012) (0.102) 9 Ophidiiformes 0.124 0.032 0.015 0.033 0.015 0.034 (0.148) (0.038) 10 Gadiformes (A) 0.211 0.133 0.173 0.043 0.082 0.048 0.015 0.212 0.111 0.078 (0.237) (0.195) (0.056) (0.100) (0.780) 11 Gadiformes (J-L) 0.021 0.013 0.017 0.004 0.008 0.005 0.003 (0.021) 0.557 0.057 0.008 (0.024) (0.006) (0.457) 12 Carangidae 0.046 0.007 0.031 0.052 0.058 0.033 0.015 0.079 0.011 (0.052) (0.034) (0.054) (0.059) (0.038) 13 Clupeiformes (J-A) 0.018 0.286 0.175 0.117 0.251 0.654 0.736 0.020 0.036 0.246 0.201 0.003 (0.146) (0.182) (0.116) (0.262) (0.679) 14 Clupeiformes (L) 0.002 0.001 0.048 0.011 0.149 15 Ichthyoplankton (L) 0.005 0.005 0.054 0.011 0.018 (0.002) 16 Scyphomedusae 0.005 17 Hydromedusae 0.005 18 Ctenophora 0.001 19 Appendicularia 0.025 0.017 0.051 0.051 (0.018) 20 Siphonophore 0.001 21 Salpida 0.010 0.008 0.007 0.001 22 Decapoda (L) 0.004 0.046 0.019 0.039 0.001 0.037 23 Euphausiacea 0.005 0.019 0.708 0.066 0.001 0.036 0.182 0.149 0.334 0.413 0.230 0.230 (0.446) 24 Chaetognatha 0.001 0.001 0.001 0.001 25 Cladocera 0.087 0.001 0.015 0.001 26 Copepoda calanoida 0.317 0.320 0.008 0.172 0.539 0.640 (0.328) (0.009) (0.125) 27 Copepoda cyclopoida 0.027 0.002 0.002 0.054 0.051 0.004 (0.016) (0.001) 28 Copepoda Nauplii (L) 0.001 0.002 0.001 0.087 0.001
29 Ciliophora 0.003 30 Micro Phytoplankton 0.050 0.005 31 Micro Flagellates 32 HNF 33 ANF 34 Bacteria 35 DOM 36 Detritus 0.001 0.001 37 Others 0.257 0.362 0.230 0.046 0.493 0.031 0.170 0.281 0.698 0.106 0.100 0.324 0.139 0.021 0.034 (0.393) (0.356) (0.263) (0.511) (0.040) (0.677) (0.217) (0.331) Sum 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 Diet data for the group 1 from = George-Nascimento et al., 1985; Naya et al., 2000, et al., 2002; Szteren et al., 2004; Suárez et al., 2005; Vallejos, 2011; 2 = Arata & Xavier, 2003, Punta et al., 2003; Arata et al., 2004; Herling et al., 2005; Suazo, 2008; 3 = Pauly et al., 1998; Sidi & Guénette, 2004; Morissette et al., 2009; 4 = Pauly et al., 1998; Morissette et al., 2009; 5 = Pauly et al., 1995; Sidi & Guénette, 2004; Morissette et al., 2009; 6 = Acuña et al., 2007; Duarte et al., 2007; 7 = Oyarzún et al., 1999, 8 = Lucas, 1982; Cassemiro et al., 2003, Moncayo-Estrada et al., 2007; Contente et al., 2011; 9 = Nyegaard et al., 2004; Chong et al., 2006; 10 = Lillo et al., 2005; Córdova et al., 2006; Tascheri et al., 2006; Saavedra et al., 2007; 11 = Pillar & Barange, 1995; Valenzuela et al., 1995; Balbontín et al., 1997; Mahe et al., 2007; 12 = Medina & Arancibia, 2002; Jardas et al., 2004; 13 = Espinoza & Bertrand, 2008; Pavés & González, 2008; Prokopchuk, 2009; 14 = Fernández & González-Quirós, 2006; Morote et al., 2008; Prokopchuk, 2009; Morote et al., 2010; Sato et al., 2011; 15 = Valenzuela et al., 1995; Balbontín et al., 1997
Annex 2. Diet composition of the pelagic subweb continuation... 16 17 18 19 20 21 22 23 24 25 26 27 28 29 31 32 34 1 Otariidae 2 Aves 3 Orcinus orca 4 Mysticeti 5 Delphinidae 6 Gempylidae 7 Sciaenidae 8 Atherinopsidae 9 Ophidiiformes 10 Gadiformes (A) 11 Gadiformes (J-L) 0.033 0.038 0.001 (0.000) 12 Carangidae 13 Clupeiformes (J-A) 14 Clupeiformes (L) 0.033 0.038 0.001 (0.002) 15 Ichthyoplankton (L) 0.130 0.190 0.008 16 Scyphomedusae 0.012 17 Hydromedusae 0.041 0.012 0.005 0.015 18 Ctenophora 0.016 0.004 19 Appendicularia 0.009 0.110 0.010 (0.009) 20 Siphonophore 0.022 21 Salpida 0.023 22 Decapoda (L) 0.222 0.253 0.086 0.100 (0.083) 23 Euphausiacea 0.041 0.111 (0.108) 24 Chaetognatha 0.003 0.010 0.061 (0.057) 25 Cladocera 0.010 0.005 0.066 0.001 26 Copepoda calanoida 0.185 0.042 0.324 0.707 0.164 0.391 0.826 0.021 (0.333) (0.713) (0.183) (0.395) (0.839) (0.020) 27 Copepoda cyclopoida 0.064 0.039 0.127 0.015 0.036 0.006 0.025 0.005 (0.017) (0.003) (0.020) (0.002) 28 Copepoda Nauplii (L) 0.001 0.100 0.003 (0.007) 29 Ciliophora 0.044 0.042 0.005 0.219 0.424 0.181 0.455 0.366 0.001 30 Micro Phytoplankton 0.358 0.005 0.350 0.228 0.017 0.467 0.216 0.160 0.210 0.244 31 Micro Flagellates 0.088 0.648 0.100 0.026 0.034 0.089 0.117 0.186 0.097 0.456 32 HNF 0.107 0.091 0.005 0.049 0.287 0.058 0.067 0.044 0.224 0.155 33 ANF 0.340 0.205 0.005 0.077 0.238 0.161 0.090 0.242 0.377 0.145 34 Bacteria 0.057 0.009 0.005 0.005 0.090 1.000 35 DOM 1.000 36 Detritus 0.050 0.014 0.050 37 Others 0.141 0.526 0.286 0.006 0.008 0.050 0.003 0.063 0.002 0.001 (0.283) (0.002) (0.060) Sum 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 16 from = Uye & Shimauchi, 2005; Hansson, 2006, Carr & Pitt, 2008; Suchman et al., 2008; 17 = Purcell & Sturdevant, 2001; Costello & Colin, 2002; 18 = Larson, 1987; Purcell & Sturdevant, 2001; Pavez et al., 2006; 19 = Vargas & González, 2004; 20 = Purcell, 1982; Purcell & Kremer, 1983; 21 = Vargas & Madin, 2004; 22 = Harms et al., 1991; Jones et al., 1997; Anger, 2001; Ariza & Ouellet, 2009; 23 = Nakagawa et al., 2001; Nakagawa et al., 2004, Schmidt et al., 2006; Pavés & González, 2008; Sánchez et al., 2011; 24 = Feigenbaum & Maris, 1984; Baier & Purcell, 1997; Giesecke & González, 2004; 25 = Atienza et al., 2006; Sánchez et al., 2011; 26 = Kleppel, 1993; Klaas et al., 2008; Vargas et al., 2008; Sánchez et al., 2011; 27 = Vargas & González, 2004; Klaas et al., 2008; Vargas et al., 2008; Nishibe et al., 2010; 28 = Meyer et al., 2002; Irigoien et al., 2003; 29 = Bernard & Rassoulzadegan, 1990; Epstein et al., 1992; Vargas & González, 2004; 31 = Jeong et al., 2010a, et al., 2010b; 32 = Boenigk & Arndt, 2002; 34 = Pavés & González, 2008 Annex 3. Landing and discards input mean for Inner Sea of Chiloe (ISCh) and Moraleda Channel (MCh) during five years (2003, 2006, 2007, 2008, 2009). The discards are only for the marine mammals and seabirds group.
Group name Clupeiforme Gillnet Long Mackerel Line hand Total s Purse- fishery line Purse-seine fishery seine fishery 1 Otariidae ISCh 0.0231 0. 2570a 0.1251 0.0031 0.1513 MCh 0.0002 0.0520 0.1382 0.00003 0.1904 2 Aves ISCh 0. 2790 b 0.2790 MCh 0.0560 0.0560 5 Delphinidae ISCh 0. 0090 c 0.0090 MCh 0.0180 0.0180 6 Gempylidae ISCh 0.6174d 0.1330 0.7504 MCh 0.0181 0.0010 0.0191 7 Sciaenidae ISCh 0.3460 0.4375e 0.0002 0.7837 MCh 0.0000 0.0000 0.0000 0.0000 8 Atherinopsidae ISCh 1.8050 0.0232 1.8282 MCh 0.5210 0.0002 0.5212 9 Ophidiiformes ISCh 1.6551 1.6551 MCh 0.4615 0.4615 10 Gadiformes ISCh 10.4220 10.4220 MCh 7.6760 7.6760 12 Carangidae ISCh 0.0042 20.3360 0.0092 20.3494 MCh 0.0031 0.1790 0.0001 0.1822 13 Clupeiformes ISCh 154.1680 3.7277 0.0010 157.8967 MCh 1.0980 0.0327 0.0000 1.1307 a = reduced value a 11% during biomass balancing (original value = 0. 2890) b = reduced value a 11% during biomass balancing (original value = 0. 3130) c = reduced value a 91% during biomass balancing (original l value = 0.1023) d = reduced value a 70% during biomass balancing (original value = 2.058) e= reduced value a 60% during biomass balancing (original value = 1.0937)
Landing information for Group 6, 7, 8, 9, 10, 12, 13, Landing i = Li * CCF / Area; Li = ton specie “i” (SERNAPESCA, 2003; SERNAPESCA, 2006; SERNAPESCA, 2007; SERNAPESCA, 2008, SERNAPESCA, 2009) for Gadiformes and Ophidiiformes, 26% of yearly total catch (SERNAPESCA, 2003; SERNAPESCA, 2006, SERNAPESCA, 2007; SERNAPESCA, 2008; SERNAPESCA, 2009) come from MCh or Inner Sea (Pool et al., 1997), for Clupeiformes data from 2003-2005 (SERNAPESCA, 2003; SERNAPESCA, 2004; SERNAPESCA, 2005); CFC = 6 2 6 2. Carbon FC (0.06 gC, Walsh, 1981); AreaMch = 8,263.0x10 m ; or AreaISCh = 9,675.0x10 m Discards information in Fisheries on Gempylidae (Acuña et al., 2007), Sciaenidae (Oyarzún et al., 1999), Atherinopsidae (Niklitschek et al., 2009), Ophidiiformes (Tascheri et al., 2003), Gadiformes (Lillo et al., 2005), Carangidae (Córdova et al., 2006; Acuña et al., 2007), Clupeiformes (Niklitschek et al., 2009). Discards information for Group 1, 2, 5 = HJPavés (unpublished data); Annex 4. Confidence intervals of the data used in the models estimated based on pedigree values. Pedigree index = 0.951.
Group name Biomas P/B Q/B Diet s 1 Otariidae 10 30 30 10 2 Aves 30 30 30 10 3 Orcinus orca 30 30 30 10 4 Mysticeti 30 30 30 10 5 Delphinidae 30 30 30 10 6 Gempylidae 50 30 30 10 7 Sciaenidae 50 30 30 10 8 Atherinopsidae 50 30 30 10 9 Ophidiiformes 50 30 30 10 10 Gad (A) 50 30 30 10 11 Gad (J-L) 80 30 30 10 12 Carangidae 50 30 30 10 13 Clup (J-A) 30 30 30 10 14 Clup (L) 30 30 30 10 15 Ichthyoplankton (L) 30 30 30 10 16 Scyphomedusae 30 30 30 10 17 Hydromedusae 10 30 30 10 18 Ctenophora 30 30 30 10 19 Appendicularia 30 30 30 10 20 Siphonophore 30 30 30 10 21 Salpida 10 30 30 10 22 Decapoda (L) 30 30 30 10 23 Euphausiacea 10 30 30 10 24 Chaetognatha 10 30 30 10 25 Cladocera 30 30 30 10 26 C. calanoida 10 30 30 10 27 C. cyclopoida 10 30 30 10 28 C. nauplii (L) 30 30 30 10 29 Ciliophora 30 30 30 10 30 Micro 10 30 30 10 phytoplankton 31 Micro flagellates 10 30 30 10 32 HNF 30 30 30 10 33 ANF 30 30 30 10 34 Bacteria 10 10 30 10 35 DOM 30 36 Detritus 10 References
Acuña, E., J.C. Villarroel, M. Araya, M. Hernández, M. Andrade, A. Cortés & J. Peñailillo, 2007. Estudio biológico-pesquero de los recursos cabinza, machuelo, sierra y blanquillo en la III y IV Regiones. Informe Final Fondo de Investigación Pesquera. FIP, Nº 2006-53. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Aguayo, A., J. Acevedo & R. Vargas, 2006. Diversidad de mamíferos marinos en las aguas del Archipiélago de los Chonos (43º39’S – 45º50’S), XI región de Chile. Ciencia y Tecnología del Mar 29: 129-145. Aguayo, M. & V. Ojeda, 1987. Estudio de la edad y crecimiento de merluza común (Merluccius gayi gayi Guichenot, 1848) (Gadiformes - Merlucciidae). Investigaciones Pesqueras (Chile) 34: 99-112. Aguayo, M., J. Chong & I. Payá, 2010. Edad, crecimiento y mortalidad natural de merluza de tres aletas, Micromesistius australis en el Océano Pacífico suroriental. Revista de Biología Marina y Oceanografía 45: 723-735. Anderson, D., 1998. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transition. In Anderson, D.M., A.D., Cembella & G.M. Hallegraeff (eds.), Physiological ecology of harmful algal blooms. Nato ASI Series G Ecological Sciences. Vol G 41: 29-48. Angelescu, V. & A. Anganuzzi, 1986. Ecología trófica de la anchoíta (Engraulidae, Engraulis anchoita) del Mar Argentino. Parte III. Requerimiento trófico individual en relación con el crecimiento, ciclo sexual y las migraciones estacionales. Revista de Investigación y Desarrollo Pesquero 5: 194-223. Anger, K., 2001. The biology of decapod crustacean larvae. Crustacean Issues 14. CRC Press. Arata, J. & J. Xavier, 2003. The diet of black-browed albatrosses at the Diego Ramirez Islands, Chile. Polar Biology 26: 638-647. Arata, J., G. Robertson, J. Valencia, J. Xavier & C. Moreno, 2004. Diet of grey-headed albatrosses at the Diego Ramírez islands, chile: ecological implications. Antarctic Sciece 16: 263-275. Ariza, P. & P. Ouellet, 2009. Diet Components of northern shrimp Pandalus borealis first stage larvae in the Northwest Gulf of St. Lawrence. Journal of Crustacean Biology 29: 532-543. Atienza, D., E. Saiz & A. Calbet, 2006. Feeding ecology of the marine cladoceran Penilia avirostris: natural diet, prey selectivity and daily ration. Marine Ecology Progress Series 315: 211-220. Baier, C.T. & J. Purcell, 1997. Trophic interactions of chaetognaths, larval fish, and zooplankton in the South Atlantic Bight. Marine Ecology Progress Series 146: 43-53. Balbontín, F., A. Llanos & V. Valenzuela, 1997. Sobreposición trófica e incidencia alimentaria en larvas de peces de Chile central. Revista Chilena de Historia Natural 70: 381-390. Bernard, C. & F. Rassoulzadegan, 1990. Bacteria or microflagellates as a major food source for marine ciliates: possible implications for the microzooplankton. Marine Ecology Progress Series 64: 147-155. Boenigk, J. & H. Arndt, 2002. Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Antonie Van Leeuwenhoek 81: 465-480. Bottrell, H.H., 1975. Generation time, length of life, instar duration and frequency of moulting, and their relationship to temperature in eight species of cladocera from the River Thames, Reading. Oecologia 19: 129-140. Bustos, C.A., M.F. Landaeta & F. Balbontín, 2008. Efectos ambientales sobre la variabilidad espacial del ictioplancton de Chile austral durante noviembre de 2005. Revista Chilena de Historia Natural 81: 205-219. Canales, T.M. & E. Leal, 2009. Parámetros de historia de vida de la anchoveta Engraulis ringens Jenyns, 1842, en la zona centro norte de Chile. Revista Biología Marina y Oceanografía 44: 173-179. Carr, E.F. & K.A. Pitt, 2008. Behavioural responses of zooplankton to the presence of predatory jellyfish. Journal of Experimental Marine Biology and Ecology 354: 101-110. Carre, C. & D. Carre, 1991. A complete life cycle of the calycophoran siphonophore Muggiaea kochi (Will) in the laboratory, under different temperature conditions: ecological implications. Philosophical Transactions: Biological Sciences 334: 27-32. Cassemiro, F.A., N.S. Hahn & T.F. Rangel, 2003. Diet and trophic ecomorphology of the silverside, Odontesthes bonariensis, of the Salto Caxias reservoir, Rio Iguaçu, Paraná, Brazil. Neotropical Ichthyology 1: 127-131 Castillo-Jordán, C., L.A. Cubillos & E., Navarro, 2010. Inter-cohort growth rate changes of common sardine (Strangomera bentincki) and their relationship with environmental conditions off central southern Chile. Fisheries Research 105: 228-236. Cauffopé, C. & S. Heymans, 2005. Energy contents and conversion factors for sea lion’s prey. In Guénette, S. & V. Christensen (eds). Food web models and data for studying fisheries and environmental impacts on Eastern Pacific ecosystems. Fisheries Centre Research Reports 13(1). University of British Columbia, Vancouver, Canada: 225-237. Cerna, F., 2003. Estimación del Crecimiento de Merluza del Sur en la Zona Sur-Austral de Chile. In Yáñez E. (ed.), Actividad Pesquera y de Acuicultura en Chile. Pontificia Universidad Catolica de Valparaiso, Valparaiso, Chile: 233-242 Cheal, A.J. & N.J. Gales, 1991. Body mass and food intake in captive, breeding bottlenose dolphins, Tursiops truncatus. Zoo Biology 10: 451-456. Chong, J.V., M. Aguayo & I. Payá, 2007. Estimación de edad, crecimiento y mortalidad natural de la merluza de cola, Macruronus magellanicus Lönnberg, 1907 (Macruronidae, Gadiformes) en el Océano Pacífico Suroriental. Revista de Biología Marina y Oceanografía 42: 311-333. Chong, J., K. Sepúlveda & C.M. Ibáñez, 2006. Variación temporal en la dieta del congrio colorado, Genypterus chilensis (Guichenot, 1881) frente al litoral de Talcahuano, Chile (36o32’S - 36o45’S). Revista de Biología Marina y Oceanografía 41: 195-202. Contente, R.F., M.F. Stefanoni & H.L. Spach, 2011. Feeding ecology of the brazilian silverside Atherinella brasiliensis (Atherinopsidae) in a sub-tropical estuarine ecosystem. Journal of the Marine Biological Association of the United Kingdom 91: 1197-1205. Córdova, J., M.A. Barbieri, M. Espejo, S. Núñez, J. Ortiz, P. Torres, F. Vejar, V. Carasti & V. Valenzuela, 2005. Evaluación hidroacústica del recurso jurel entre la V y X Regiones, invierno, año 2004. Informe Final Fondo de Investigación Pesquera. FIP N° 2004-06. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Córdova, J., R. Céspedes, V. Ojeda, F. Balbontín, P. Rojas, A. Saavedra, M.A. Barbieri & J.C. Saavedra, 2006. Evaluación del stock desovante de merluza del sur y merluza de cola en la zona sur austral, año 2005 (2da. Licitación). Informe Final Fondo de Investigación Pesquera. FIP N° 2005-04. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Costello, J.H. & S.P. Colin, 2002. Prey resource use by coexistent hydromedusae from Friday Harbor, Washington. Limnology and Oceanography 47: 934-942. Cubillos, L.A. & H. Arancibia, 1995. Comparative growth performance of horse mackerel of the genus Trachurus, with emphasis on T. symmetricus murphyi in Chile. Scientia Marina 59: 647-652. Cubillos, L.A., P. Ruiz, G. Claramunt, S. Gacitúa, S. Núñez, L.R. Castro, K. Riquelme, C. Alarcón, C. Oyarzún & A. Sepúlveda, 2007. Spawning, daily egg production, and spawning stock biomass estimation for common sardine (Strangomera bentincki) and anchovy (Engraulis ringens) off central southern Chile in 2002. Fisheries Research 86: 228-240. Deibel, D. & B. Lowen, 2011. A review of the life cycles and life-history adaptations of pelagic tunicates to environmental conditions. ICES Journal of Marine Science, doi:10.1093/icesjms/fsr159. Dolana, J.R. & D.W. Coats, 1990. seasonal abundances of planktonic ciliates and microflagellates in mesohaline Chesapeake Bay Waters. Estuarine Coastal and Shelf Science 31: 157-175. Drebes, G., S. F. Kuhn, A. Gmelch & E. Schnepf, 1996. Cryothecomonas aesfivalis sp. nov., a colourless nanoflagellate feeding on the marine centric diatom Guinardia delicatula (Cleve) Hasle. Helgoland Marine Research 50: 497-515. Duarte, F., C.M. Ibáñez & J. Chong, 2007. Cambios en la morfometría bucal y su relación con la dieta de Thyrsites atun (Euphrasen, 1791) en el centro-sur de Chile. Revista Chilena de Historia Natural 80: 407-417. Durbin, E.G., A.G. Durbin, R.W. Langton & R.E. Bowman, 1983. Stomach contents of silver hake, Merluccius biunearis, and atlantic cod, Gadus morhua, and estimation of their daily rations. Fishery Bulletin 81: 437-454. Epstein, S.S., I.V. Burkovsky & M.P. Shiaris, 1992. Ciliate grazing on bacteria, flagellates, and microalgae in a temperate zone sandy tidal flat: ingestion rates and food niche partitioning. Journal of Experimental Marine Biology and Ecology 165: 103-123. Espinoza, P. & A. Bertrand, 2008. Revisiting Peruvian anchovy (Engraulis ringens) trophodynamics provides a new vision of the Humboldt Current system. Progress in Oceanography 79: 215-227. Feigenbaum, D.L. & R.C. Maris, 1984. Feeding in the Chaetognatha. Oceanography and Marine Biology: Annual Review 22: 343-392. Gabrielsen, G.W. & B. Brekke, 1994. Assimilation efficiency of adult kittiwakes and brunnich guillemots fed capelin and arctic cod. Polar Biology 14: 279-284. George-Nascimento, M., R. Bustamante & C. Oyarzun, 1985. Feeding ecology of the South American sea lion Otaria flavescens: food contents and food selectivity. Marine Ecology-Progress Series 21: 135-143. Giesecke, R. & H.E. González, 2004. Feeding of Sagitta enflata and vertical distribution of chaetognaths in relation to low oxygen concentrations. Journal of Plankton Research 26: 475 -486. Giesecke, R. & H. E. González, 2008. Reproduction and feeding of Sagitta enflata in the Humboldt Current system off Chile. ICES Journal of Marine Science 65: 361–370. González, H.E., M. Sobarzo, D. Figueroa & E.M. Nthig, 2000. Composition, biomass and potential grazing impact of the crustacean and pelagic tunicates in the northern Humboldt Current area off Chile: differences between El Niño and non-El Niño years. Marine Ecology-Progress Series 195: 201-220. González, H.E., M.J. Calderón, L. Castro, A. Clement, L.A. Cuevas, G. Daneri, J.L. Iriarte, L. Lizrraga, R. Martnez, E. Menschel, N. Silva, C. Carrasco, C. Valenzuela, C.A. Vargas & C. Molinet, 2010. Primary production and plankton dynamics in the Reloncaví Fjord and the Interior Sea of Chiloé, Northern Patagonia, Chile. Marine Ecology-Progress Series 402: 13-30. González, H.E., L. Castro, G. Daneri, J.L. Iriarte, N. Silva, C.A. Vargas, R. Giesecke, & N. Sánchez, 2011. Seasonal plankton variability in Chilean Patagonia fjords: Carbon flow through the pelagic food web of Aysen Fjord and plankton dynamics in the Moraleda Channel basin. Continental Shelf Research 31: 225- 243. Govoni, J.J., G.W. Boehlert & Y. Watanabe, 1986. The physiology of digestion in fish larvae. Environmental Biology of Fishes 16: 59-77. Gru, D. & M. Cousseau, 1982. Estudio de edad y crecimiento de la sardina fueguina (Sprattus fuegensis) de las costas de la provincia de Santa Cruz e Islas Malvinas. Instituto Nacional de Investigación y Desarrollo Pesquero 3: 51-58. Hain, J., M. Hyman, R. Kennedy & H. Winn, 1985. The role of cetaceans in the shelf- edge region of the northeastern United States. Marine Fisheries Review 47: 13- 17. Hansson, L., 2006. A method for in situ estimation of prey selectivity and predation rate in large plankton, exemplified with the jellyfish Aurelia aurita (L.). Journal of Experimental Marine Biology and Ecology 328: 113-126. Hamame, M. & T. Antezana, 2010. Vertical diel migration and feeding of Euphausia vallentini within southern Chilean fjords. Deep-Sea Research Part I- Oceanographic Research Papers 57: 642–651. Harms, J., K., Anger, S., Klaus & B. Seeger, 1991. Nutritional effects on ingestion rate, digestive enzyme activity, growth, and biochemical composition of Hyas araneus L. (Decapoda: Majidae) larvae. Journal of Experimental Marine Biology and Ecology 145: 233–265. Herling, C., B.M., Culik & J.C. Hennicke, 2005. Diet of the Humboldt penguin (Spheniscus humboldti) in northern and southern Chile. Marine Biology 147: 13–25. Hirst, A., J., Roff & R. Lampitt, 2003. A synthesis of growth rates in marine epipelagic invertebrate zooplankton. Advances in Marine Biology 44: 1–142. Horn, P.L., 2002. Age estimation of barracouta (Thyrsites atun) off southern New Zealand. Mar. Freshwater Research 53: 1169–1178. Houde, E., 1989. Comparative Growth, Mortality, and Energetics of Marine Fish Larvae: Temperature and Implied Latitudinal Effects. Fishery Bulletin 87: 471– 495. Hucke-Gaete, R., R., Álvarez, M., Navarro, J., Ruiz, P., Lo Moro & A. Farías, 2010. Investigación para Desarrollo de Área Marina Costera Protegida Chiloé-Palena- Guaitecas. Informe Final de estudio financiado por FNDR - Bid Turismo Cód. BIP N ° 30040215-0, Gobierno Regional de Los Lagos. Unidad técnica mandante: CONAMA. Puerto Montt, Chile. Huntley, M.E. & M.D.G. Lopez, 1992. Temperature-Dependent Production of Marine Copepods: A Global Synthesis. The American Naturalist 140: 201–242. Ikeda, T.& N. Shiga, 1999. Production, metabolism and production/biomass (P/B) ratio of Themisto japonica (Crustacea : Amphipoda) in Toyama Bay, southern Japan Sea. Journal of Plankton Research 21: 299–308. Irigoien, X., J., Titelman, R.P., Harris, D., Harbour & C. Castellani, 2003. Feeding of Calanus finmarchicus nauplii in the Irminger Sea. Marine Ecology Progress Series 262: 193–200. Jardas, I., M., Šantić & A. Pallaoro, 2004. Diet composition and feeding intensity of horse mackerel, Trachurus trachurus (Osteichthyes: Carangidae) in the eastern Adriatic. Marine Biology 144, 1051–1056. Jeong, H.J., Y.D. Yoo, N.S. Kang, J.R. Rho, K.A. Seong, J.W. Park, G.S. Nam & W. Yih, 2010a. Ecology of Gymnodinium aureolum. I. Feeding in western Korean waters. Aquatic Microbial Ecology 59:239–255. Jeong, H. J., Y. D. Yoo, J. S. Kim, K. A. Seong, N. S. Kang & T. H. Kim, 2010b. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Science Journal 45:65– 91. Jones, D. A., M. Kumlu, L. Le Vay & D. J. Fletcher, 1997. The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: A review. Aquaculture 155:285–295. Karlou-Riga, C. & A. Sinis, 1997. Age and growth of horse mackerel, Trachurus trachurus (L.), in the Gulf of Saronikos (Greece). Fisheries Research 32:157– 171. Kastelein, R. A., J. Kershaw, E. Berghout & P. R. Wiepkema, 1995. The food consumption of South American sea lions (Otaria flavescens). Aquatic Mammals 21:43–53. Kenney, R., G. Scott, T. Thompson & H. Winn, 1997. Estimates of prey consumption and trophic impacts of cetaceans in the usa northeast continental shelf ecosystem. Journal of Northwest Atlantic Fishery Science 22:155–171. Klaas, C., P. G. Verity & S. Schultes, 2008. Determination of copepod grazing on natural plankton communities: correcting for trophic cascade effects. Marine Ecology Progress Series 357:195–206. Kleppel, G., 1993. On the diets of calanoid copepods. Marine Ecology Progress Series 99:183–195. Landaeta, M. & L. Castro, 2006. Variabilidad estacional en los patrones espaciales de las asociaciones ictioplanctonicas de la zona de fiordos de Chile Austral. Ciencia y Tecnologia del Mar 29:107–127. Larson, R. J., 1986. Seasonal changes in the standing stocks, growth rates, and production rates of gelatinous predators in Saanich Inlet, British Columbia. Marine Ecology Progress Series 33:89–98. Larson, R. J., 1987. Daily ration and predation by medusae and ctenophores in Saanich Inlet, B.C., Canada. Netherlands Journal of Sea Research 21:35–44. Lillo, S., R. Céspedes, M. Barbieri, A. Saavedra & P. Gálvez, 2004. Programa temporal de evaluación hidroacústica de merluza del sur en aguas interiores de la X y XI Regiones, año 2002. Informe Final Fondo de Investigación Pesquera. FIP Nº 2002-07. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Lillo, S., R. Céspedes, V. Ojeda, F. Balbontín, R. Bravo, A. Saavedra, M.A. Barbieri & C. Vera, 2005. Evaluación del stock desovante de merluza del sur y merluza de cola en la zona sur austral, año 2004. Informe Final Fondo de Investigación Pesquera. FIP 2004-07. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Lillo, S., R. Céspedes, M. Barbieri, R. Meléndez & V. Ojeda 2006. Programa temporal de evaluación hidroacústica de merluza del sur en aguas interiores de la X y XI Regiones, año 2004. Informe Final Fondo de Investigación Pesquera. FIP 2004- 40. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Lillo, S., E. Molina, C. Lang, V. Ojeda, R. Céspedes, L. Adasme, R. Meléndez, M. Rojas & A. Saavedra, 2008. Evaluación hidroacústica de merluza del sur en aguas interiores de la X y XI Regiones, año 2006. Informe Final Fondo de Investigación Pesquera. FIP Nº 2006-10. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. López-Urrutia, Á., X. Irigoien, J. L. Acuña & R. Harris, 2003. In situ feeding physiology and grazing impact of the appendicularian community in temperate waters. Marine Ecology Progress Series 252:125–141. Lucas, J. R., 1982. Feeding ecology of the Gulf silverside, Menidia peninsulae, near Crystal River, Florida, with notes on its life history. Estuaries 5:138–144. Lynch, M., 1980. The evolution of Cladoceran life histories. The Quarterly Review of Biology 55:23–42. Macpherson, E., 1983. Feeding pattern of the kingklip (Genypterus capensis) and its effect on the hake (Merluccius capensis) resource off the coast of Namibia. Marine Biology 78:105–112. Mahe, K., R. Amara, T. Bryckaert, M. Kacher & J. M. Brylinski, 2007. Ontogenetic and spatial variation in the diet of hake (Merluccius merluccius) in the Bay of Biscay and the Celtic Sea. ICES Journal of Marine Science 64:1210 –1219. Marshalonis, D. & J. L. Pinckney, 2008. Grazing and assimilation rate estimates of hydromedusae from a temperate tidal creek system. Hydrobiologia 606:203– 211. McGurk, M. D., 1984. Effects of delayed feeding and temperature on the age of irreversible starvation and on the rates of growth and mortality of Pacific herring larvae. Marine Biology 84:13–26. Medina, M. & H. Arancibia, 2002. Dinámica trófica del jurel (Trachurus symmetricus murphyi) en el norte de Chile. Investigaciones Marinas 30:45–55. Medina, M., H. Arancibia, & S. Neira, 2007. Un modelo trófico preliminar del ecosistema pelágico del norte de Chile (18°20S - 24°00S). Investigaciones marinas 35:25–38. Melgo, J.L., L. Morissette, K. Kaschner & L. Gerber, 2009. Food web model and data for studying the interactions between marine mammals and fisheries in the Caribbean ecosystem, in: Morissette, L., J.L. Melgo, K. Kaschner & L.R. Gerber (eds.), Modelling the trophic role of marine mammals in tropical areas: data requirements, uncertainty, and validation. Fisheries Centre Research Reports 17(2). Fisheries Centre. University of British Columbia. Vancouver, Canada: 48- 107. Meyer, B., X. Irigoien, M. Graeve, R. Head & R. Harris, 2002. Feeding rates and selectivity among nauplii, copepodites and adult females of Calanus finmarchicus and Calanus helgolandicus. Helgoland Marine Research 56:169– 176. Moncayo-Estrada, R., C. Escalera-Gallardo, C. López & O. T. Lind, 2007. Diet of Chirostoma lucius (Pisces: Atherinomorpha): seasonal trophic spectrum and ontogeny of piscivory. The Southwestern Naturalist 52:229–233. Morissette, L., J.L. Melgo, K. Kaschner & L. Gerber, 2009. Modelling the trophic role of marine mammals in tropical areas: data requirements, uncertainty, and validation. Fisheries Centre Research Reports. Fisheries Centre. University of British Columbia, 17 (2). Vancouver, Canada. Morissette, L., K. Kaschner & L. R. Gerber, 2010. Ecosystem models clarify the trophic role of whales off Northwest Africa. Marine Ecology Progress Series 404:289– 302. Morote, E., M. P. Olivar, F. Villate & I. Uriarte, 2008. Diet of round sardinella, Sardinella aurita, larvae in relation to plankton availability in the NW Mediterranean. Journal of Plankton Research 30:807–816. Morote, E., M. P. Olivar, F. Villate & I. Uriarte, 2010. A comparison of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) larvae feeding in the Northwest Mediterranean: influence of prey availability and ontogeny. ICES Journal of Marine Science: Journal du Conseil 67:897 –908. Mujica, A., 2008. Crustáceos decápodos planctónicos de los canales de la XI región. Ciencia y Tecnologia del Mar 31:97–108. Mujica, A. & M. Nava, 2010. Distribución espacial de larvas de crustáceos decápodos planctónicos en canales orientales de la isla Chiloé, Chile. Latin American Journal of Aquatic Research 38:95–106. Mujica, A., M. Nava & A. Araya, 2011. Larvas de Sergestes arcticus Kroyer, 1855, Neotrypaea uncinata (H. Milne-Edwards, 1837) y Munida gregaria (Fabricius, 1793), entre el seno Reloncaví y Boca del Guafo, sur de Chile. Latin American Journal of Aquatic Research 39:33–42. Munuera Fernández, I. & R. González-Quirós, 2006. Analysis of feeding of Sardina pilchardus (Walbaum, 1792) larval stages in the central Cantabrian Sea. Scientia Marina 70:131–139. Nakagawa, Y., Y. Endo & K. Taki, 2001. Diet of Euphausia pacifica Hansen in Sanriku waters off northeastern Japan. Plankton Biology and Ecology 48:68–77. Nakagawa, Y., T. Ota, Y. Endo, K. Taki & H. Sugisaki, 2004. Importance of ciliates as prey of the euphausiid Euphausia pacifica in the NW North Pacific. Marine Ecology Progress Series 271:261–266. Naya, D., R. Vargas & Arim, 2000. Análisis preliminar de la dieta del león marino del sur (Otaria flavescens) en Isla de Lobos, Uruguay. Boletín de la Sociedad de Zoología del Uruguay 12: 14-21. Naya, D. E., M. Arim & R. Vargas, 2002. Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos, Uruguay. Marine Mammal Science 18:734–745. Neira, S. & H. Arancibia, 2004. Trophic interactions and community structure in the upwelling system off Central Chile (33-39°S). Journal of Experimental Marine Biology and Ecology 312:349–366. Neira, S., H. Arancibia & L. Cubillos, 2004. Comparative analysis of trophic structure of commercial fishery species off Central Chile in 1992 and 1998. Ecological Modelling 172:233–248. Newbury, T. K., 1978. Consumption and growth rates of chaetognaths and copepods in subtropical oceanic waters. Pacific Science 32:61–78. Niklitschek, E., P. Toledo, E. Hernández, J. Nelson, M. Soule, C. Herranz, C. Murillo & X. Valenzuela, 2009 Identificación y evaluación hidroacústica de pequeños pelágicos en aguas interiores de la X y XI regiones, año 2007. Informe Final Fondo de Investigación Pesquera. FIP Nº 2007-05. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Nishibe, Y., T. Kobari & T. Ota, 2010. Feeding by the cyclopoid copepod Oithona similis on the microplankton assemblage in the Oyashio region during spring. Plankton and Benthos Research 5:74–78. Nyegaard, M., A. Arkhipkin & P. Brickle, 2004. Variation in the diet of Genypterus blacodes (Ophidiidae) around the Falkland Islands. Journal of Fish Biology 65:666–682. Oporto, J., L. Brieva, R. Navarro, A. Turner, C. Espinoza, H. Paves & O. Mora, 1999. Cuantificación poblacional de lobos marino en la X y XI regiones. Informe Final Fondo de Investigación Pesquera. FIP Nº 97-44. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Oyarzún, C., N. Cortés, J. Chong, H. Arancibia, M. Landaeta & A. Pinto, 1999. Estudio Biologico Pesquero de la corvina en la zoa centro-sur. Informe Final Fondo de Investigación Pesquera. FIP Nº 97-19. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Palma, S., P. Apablaza & D. Soto, 2007. Diversity and aggregation areas of planktonic cnidarians of the southern channels of Chile (Boca del Guafo to Pulluche Channel). Investigaciones Marinas 35: 71-82. Palma, S., N. Silva, M. Cristina Retamal & L. Castro, 2011. Seasonal and vertical distributional patterns of siphonophores and medusae in the Chiloé Interior Sea, Chile. Continental Shelf Research 31:260–271. Palomares, M. & D. Pauly, 2011. SeaLifeBase. World Wide Web electronic publication. www.sealifebase.org, version (08/2011), online, dataset, http://www.sealifebase.org. Pauly, D., A. Trites, E. Capuli & V. Christensen, 1995. Diet composition and trophic levels of marine mammals. ICES Council Meeting Papers 13: 1-22. Pauly, D., A. W. Trites, E. Capuli & V. Christensen. 1998. Diet composition and trophic levels of marine mammals. ICES Journal of Marine Science 55:467 –481 Pavez, M. A., L. R. Castro & H. E. González, 2006. Across-shelf predatory effect of Pleurobrachia bachei (Ctenophora) on the small-copepod community in the coastal upwelling zone off northern Chile (23° S). Journal of Plankton Research 28:115 –129. Pavez, P., G. Plaza, V. Espejo, B. Dyer, H. Cerisola, J. Saavedra, V. Almanza & M. Matamala, 2008. Estudio Biológico-Pesquero del pejerrey de mar X Región. Informe Final Fondo de Investigación Pesquera. FIP Nº 2006-58. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Pavés, H. J. & H. E. González, 2008. Carbon fluxes within the pelagic food web in the coastal area off Antofagasta (23°S), Chile: The significance of the microbial versus classical food webs. Ecological Modelling 212:218–232. Pearre, S.J., 1991. Growth and reproduction. In Bone, Q., H. Kapp & A.C. Pierrot-Bults (eds), The Biology of Chaetognaths. Oxford University Publs, New York: 61- 75. Pillar, S. C. & M. Barange, 1995. Diel feeding periodicity, daily ration and vertical migration of juvenile Cape hake off the west coast of South Africa. Journal of Fish Biology 47:753–768. Pinchuk, A. I. & R. R. Hopcroft, 2006. Seasonal variations in the growth rates of euphausiids (Thysanoessa inermis, T. spinifera, and Euphausia pacifica) from the northern Gulf of Alaska. Marine Biology 151:257–269. Pool, H., F. Balbontin, C. Montenegro, N. Cortes & M. Arriaza, 1997. Interacciones Tróficas en Recursos Demersales en la Zona Sur-Austral. Informe Final Fondo de Investigación Pesquera. FIP IT 94-32. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Prenski L.B. & V. Angelescu, 1993. Ecología trófica de la merluza común (Merluccius hubbsi) del Mar Argentino. Parte 3. Consumo anual de alimento a nivel poblacional y su relación con la explotación de las pesquerías multiespecíficas. Mar del Plata: Instituto Nacional de Investigación y Desarrollo Pesquero 1: 1- 118. Preuss, T. G., M. Hammers-Wirtz, U. Hommen, M. N. Rubach & H. T. Ratte, 2009. Development and validation of an individual based Daphnia magna population model: The influence of crowding on population dynamics. Ecological Modelling 220:310–329. Prokopchuk, I., 2009. Feeding of the Norwegian spring spawning herring Clupea harengus (Linne) at the different stages of its life cycle. Deep Sea Research Part II: Topical Studies in Oceanography 56:2044–2053. Punta, G., P. Yorio & G. Herrera, 2003. Temporal patterns in the diet and food partitioning in imperial cormorants (Phalacrocorax atriceps) and Rock Shags (P. Magellanicus) breeding at Bahía Bustamante, Argentina. The Wilson Bulletin 115:307–315. Purcell, J. E. 1981. Dietary composition and diel feeding patterns of epipelagic siphonophores. Marine Biology 65:83–90. Purcell, J. E., 1982. Feeding and growth of the siphonophore Muggiaea atlantica (Cunningham 1893). Journal of Experimental Marine Biology and Ecology 62:39–54. Purcell, J. E., 1983. Digestion rates and assimilation efficiencies of siphonophores fed zooplankton prey. Marine Biology 73:257–261. Purcell, J. E. & P. Kremer, 1983. Feeding and metabolism of the siphonophore Sphaeronectes gracilis. Journal of Plankton Research 5: 95–106. Purcell, J. E. & M. V. Sturdevant, 2001. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Marine Ecology Progress Series 210:67–83. Pérez-Barros, P., S. Thatje, J. A. Calcagno & G. A. Lovrich, 2007. Larval development of the subantarctic squat lobster Munida subrugosa (White, 1847) (Anomura: Galatheidae), reared in the laboratory. Journal of Experimental Marine Biology and Ecology 352:35–41. Redalje, D. G. & E. A. Laws, 1981. A new method for estimating phytoplankton growth rates and carbon biomass. Marine Biology 62:73–79. Reeve, M. R., M. A. Walter & T. Ikeda, 1978. Laboratory studies of ingestion and food utilization in lobate and tentaculate ctenophores. Limnology and Oceanography 23:740–751. Ross, R.M., 1982. Energetics of Euphausia pacifica. II. Complete carbon and nitrogen budgets at 8º and 12º~ throughout the life span. Marine Biology 68: 15-23 Rosen, D., L. Williams & A. Trites, 2000. Effect of ration size and meal frequency on assimilation and digestive efficiency in yearling Steller sea lions, Eumetopias jubatus. Aquatic Mammals 26:76–82. SERNAPESCA, 2003. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2004. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2005. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2006. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2007. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2008. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. SERNAPESCA, 2009. Anuario Estadístico de Pesca (Servicio Nacional de Pesca. Valparaiso, Chile), online, http://www.sernapesca.cl. Saavedra, A., V. Correa, R. Cespedes, V. Ojeda, L. Adasme, E. Diaz, J. Oliva & P. Rojas, 2007. Evaluación hidroacústica del stock parental de merluza de tres aletas en su unidad de pesquería, año 2005. Informe Final Fondo de Investigación Pesquera. FIP N° 2005-06. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Santos, M. B., M. R. Clarke & G. J. Pierce, 2001. Assessing the importance of cephalopods in the diets of marine mammals and other top predators: problems and solutions. Fisheries Research 52:121–139. Sato, N., D. Hernández & M. Viñas, 2011. Hábitos alimentarios de las larvas de Engraulis anchoita (Hubbs & Marini, 1935) en las aguas costeras de la Provincia de Buenos Aires, Argentina. Latin American Journal of Aquatic Research 39:16–24. Sato, R., Y. Ishibashi, Y. Tanaka, T. Ishimaru & M. J. Dagg, 2008. Productivity and grazing impact of Oikopleura dioica (Tunicata, Appendicularia) in Tokyo Bay. Journal of Plankton Research 30:299 –309. Scheinberg, R. D., M. R. Landry & A. Calbet, 2005. Grazing of two common appendicularians on the natural prey assemblage of a tropical coastal ecosystem. Marine Ecology Progress Series 294:201–212. Schmidt, K., A. Atkinson, K.-J. Petzke, M. Voss & D. W. Pond, 2006. protozoans as a food source for antarctic krill, Euphausia superba: complementary insights from stomach content, fatty acids, and stable isotopes. Limnology and Oceanography 51:2409–2427. Shaw, T. C., W. T. Peterson & L. R. Feinberg, 2010. Growth of Euphausia pacifica in the upwelling zone off the Oregon coast. Deep Sea Research Part II: Topical Studies in Oceanography 57:584–593. Shenker, J. M., 1985. Carbon content of the neritic scyphomedusa Chrysaora fuscescens. Journal of Plankton Research 7:169 –173. Sidi, M. & M. Guénette, 2004. Modèle trophique de la ZEE mauritanienne: comparaison de deux périodes (1987 et 1998), in: Palomares, M.L.D., Pauly, D. (eds), West African marine ecosystems: models and fisheries impacts. Fisheries Centre Research Reports (12)7. University of British Columbia, Vancouver, Canada: 12-38. Sielfeld, W., C. Guerra, L. Durán, E. Acuña, A. Aguayo-Lobo, M. Sepúlveda, F. Palma, A. Malinarich, G. Cerda, A. Bolvarán, R. Grau, X. Veloso, Y. Guerra, M. Vargas, N. Amado, R. Peredo & J. Galaz, 1997. Monitoreo de la pesqueria y censo del lobo marino comun en el litoral de la I a IV regiones. Informe Final Fondo de Investigación Pesquera. FIP, Nº 95-28. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Stoecker, D. K., D. J. Gifford & M. Putt, 1994. Preservation of marine planktonic ciliates: losses and cell shrinkage during fixation. Marine Ecology Progress Series 110:293–299. Strom, S. L. & T. A. Morello, 1998. comparative growth rates and yields of ciliates and heterotrophic dinoflagellates. Journal of Plankton Research 20:571–584. Suarez, A., D. Sanfelice, M. Cassini & H. L. Cappozzo, 2005. Composition and seasonal variation in the diet of the South American Sea Lion (Otaria flavescens) from Quequén, Argentina. Latin American Journal of Aquatic Mammals 4:163–174. Suazo, C.G., 2008. Black-browed albatross foraging on jellyfish prey in the southeast Pacific coast, southern Chile. Polar Biology 31: 755-757. Suchman, C. L., E. A. Daly, J. E. Keister, W. T. Peterson & R. D. Brodeur. 2008. Feeding patterns and predation potential of scyphomedusae in a highly productive upwelling region. Marine Ecology Progress Series 358:161–172. Szteren, S., D. Naya & M. Arim. 2004. Overlap between pinniped summer diet and artisanal fishery catches in Uruguay. Latin American Journal of Aquatic Mammals 3:119–125. Sánchez, N., H. E. González & J. L. Iriarte, 2011. Trophic interactions of pelagic crustaceans in Comau Fjord (Chile): their role in the food web structure. Journal of Plankton Research 33:1212 –1229. Tascheri, R., J. Sateler, J. Merino, E. Diaz, V. Ojeda & M. Montecinos, 2003. Estudio biológico-pesquerocongrio colorado, congrio negro y congrio dorado enla zona centro-sur. Informe Final Fondo de Investigación Pesquera. FIP N° 2001-15. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Tascheri, R., J. Sateler, H. Rebolledo, R. Alarcón, L. Bustos, P. Barraza, S. Núñez, A. Sepúlveda & J. González, 2006. Monitoreo de las Capturas de merluza común, Año 2005. Informe Final Fondo de Investigación Pesquera. FIP N° 2005-07. Subsecretaría de Pesca, Valparaíso, Chile. http://www.fip.cl. Uye, S. & H. Shimauchi, 2005. Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. Journal of Plankton Research 27:237 –248. Uye, S., 1982. Length-weight relationships of important zooplankton from the Inland Sea of Japan. Journal of the Oceanographical Society of Japan 38: 149-158. Uye, S. & K. Sano, 1998. Seasonal variations in biomass, growth rate and production rate of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Marine Ecology Progress Series 163:37–44. Valenzuela, V., F. Balbontin & A. Llanos, 1995. Composicion de la dieta y tamanio de las presas de los estadios larvales de ocho especies de peces de la costa central de Chile. Revista de Biologia Marina 30: 275-291. Vallejos, A., 2011. Dieta del lobo fino austral, Arctocephalus australis (Zimmermann, 1783), en Isla Guafo, Chile. Marine Biologist thesis: Universidad de Los Lagos. Osorno. Chile. Vargas, C.A. & H.E. González, 2004a. Plankton community structure and carbon cycling in a coastal upwelling system. I. Bacteria, microprotozoans and phytoplankton in the diet of copepods and appendicularians. Aquatic Microbial Ecology 34: 151-164. Vargas, C. A. & H.E. González. 2004b. Plankton community structure and carbon cycling in a coastal upwelling system. II. Microheterotrophic pathway. Aquatic Microbial Ecology 34:165–180. Vargas, C. A. & L. P. Madin, 2004. Zooplankton feeding ecology: clearance and ingestion rates of the salps Thalia democratica, Cyclosalpa affinis and Salpa cylindrica on naturally occurring particles in the Mid-Atlantic Bight. Journal of Plankton Research 26:827 –833. Vargas, C. A., R. A. Martnez, H. E. Gonzlez & N. Silva, 2008. Contrasting trophic interactions of microbial and copepod communities in a fjord ecosystem, Chilean Patagonia. Aquatic Microbial Ecology 53:227–242. Viddi, F. A., R. Hucke-Gaete, J. P. Torres-Florez & S. Ribeiro, 2010. Spatial and seasonal variability in cetacean distribution in the fjords of northern Patagonia, Chile. ICES Journal of Marine Science 67: 959–970. Villenas, F., D. Soto, & S. Palma. 2009. Cambios interanuales en la biomasa y biodiversidad de zooplancton gelatinoso en aguas interiores de Chiloé, sur de Chile (Primavera 2004 y 2005). Revista de Biología Marina y Oceanografía 44: 309-324. Walline, P. 1987, Growth and ingestion rates of larval fish populations in the coastal waters of Israel. Journal of Plankton Research 9:91 –102. Walsh, J. J., 1981. A carbon budget for overfishing off Peru. Nature 290:300–304. Wiff, R., V. Ojeda & J. C. Quiroz, 2007. Age and growth in pink cusk-eel (Genypterus blacodes) off the Chilean austral zone: evaluating differences between management fishing zones. Journal of Applied Ichthyology 23:270–272. Williams, T.M., J.A. Estes, D.F. Doak & A.M. Springer, 2004. Killer appetites: assessing the role of predators in ecological communities. Ecology 85: 3373- 3384. Withell, A. & J. Wankowski, 1989. Age, growth estimates for pink ling, Genypterus blacodes (Schneider), and gemfish, Rexea solandri (Cuvier), from Eastern Bass Strait, Australia. Marine & Freshwater Research. 40: 215-226. Zamorano-Abramson, J., J. Gibbons & J. Capella, 2010. Diversity and summer distribution of cetaceans in inlet waters of northern Aisén, Chile. Anales del Instituto de la Patagonia 38: 151-157.