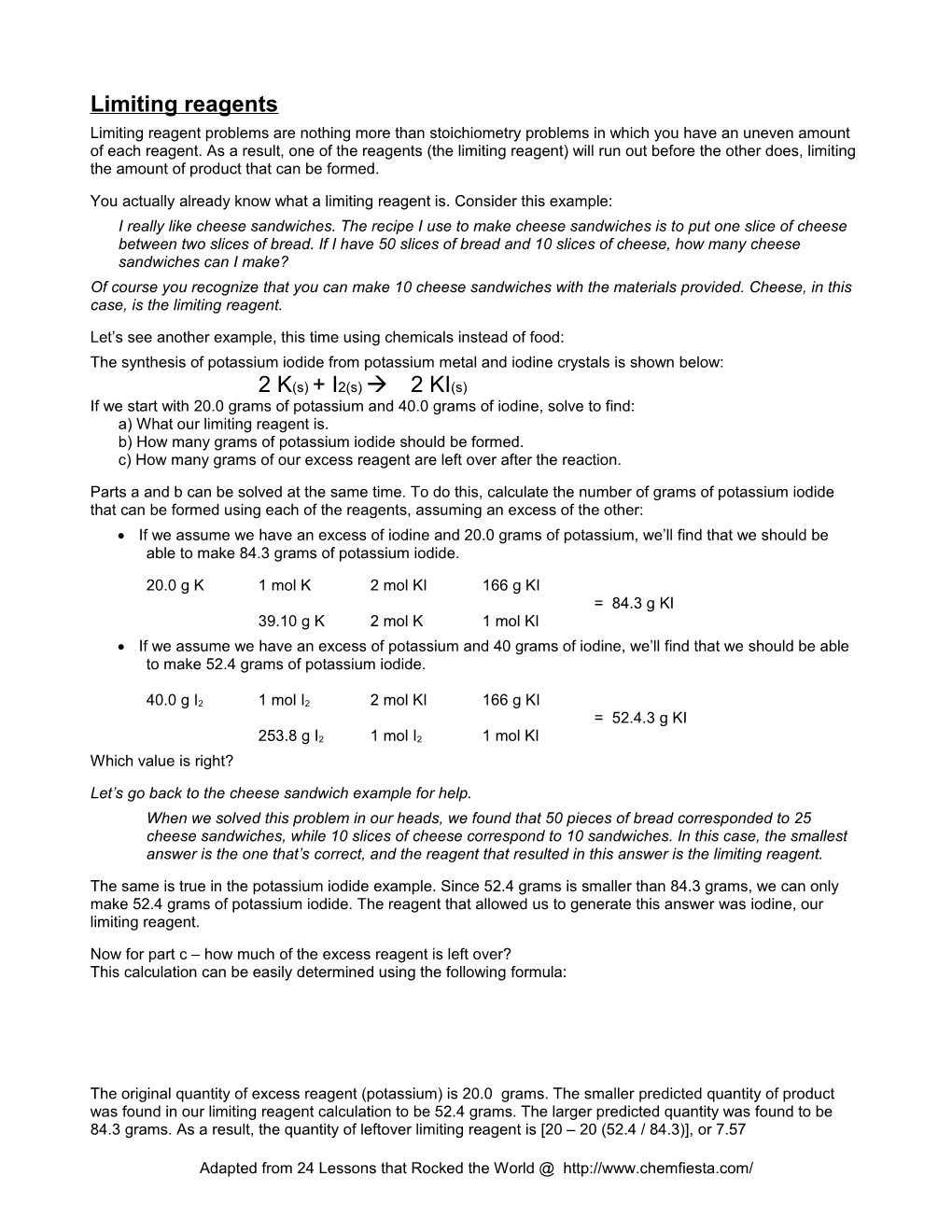
Limiting reagents Limiting reagent problems are nothing more than stoichiometry problems in which you have an uneven amount of each reagent. As a result, one of the reagents (the limiting reagent) will run out before the other does, limiting the amount of product that can be formed.
You actually already know what a limiting reagent is. Consider this example: I really like cheese sandwiches. The recipe I use to make cheese sandwiches is to put one slice of cheese between two slices of bread. If I have 50 slices of bread and 10 slices of cheese, how many cheese sandwiches can I make? Of course you recognize that you can make 10 cheese sandwiches with the materials provided. Cheese, in this case, is the limiting reagent.
Let’s see another example, this time using chemicals instead of food: The synthesis of potassium iodide from potassium metal and iodine crystals is shown below: 2 K(s) + I2(s) 2 KI(s) If we start with 20.0 grams of potassium and 40.0 grams of iodine, solve to find: a) What our limiting reagent is. b) How many grams of potassium iodide should be formed. c) How many grams of our excess reagent are left over after the reaction.
Parts a and b can be solved at the same time. To do this, calculate the number of grams of potassium iodide that can be formed using each of the reagents, assuming an excess of the other: If we assume we have an excess of iodine and 20.0 grams of potassium, we’ll find that we should be able to make 84.3 grams of potassium iodide.
20.0 g K 1 mol K 2 mol KI 166 g KI = 84.3 g KI 39.10 g K 2 mol K 1 mol KI If we assume we have an excess of potassium and 40 grams of iodine, we’ll find that we should be able to make 52.4 grams of potassium iodide.
40.0 g I2 1 mol I2 2 mol KI 166 g KI = 52.4.3 g KI 253.8 g I2 1 mol I2 1 mol KI Which value is right?
Let’s go back to the cheese sandwich example for help. When we solved this problem in our heads, we found that 50 pieces of bread corresponded to 25 cheese sandwiches, while 10 slices of cheese correspond to 10 sandwiches. In this case, the smallest answer is the one that’s correct, and the reagent that resulted in this answer is the limiting reagent.
The same is true in the potassium iodide example. Since 52.4 grams is smaller than 84.3 grams, we can only make 52.4 grams of potassium iodide. The reagent that allowed us to generate this answer was iodine, our limiting reagent.
Now for part c – how much of the excess reagent is left over? This calculation can be easily determined using the following formula:
The original quantity of excess reagent (potassium) is 20.0 grams. The smaller predicted quantity of product was found in our limiting reagent calculation to be 52.4 grams. The larger predicted quantity was found to be 84.3 grams. As a result, the quantity of leftover limiting reagent is [20 – 20 (52.4 / 84.3)], or 7.57
Adapted from 24 Lessons that Rocked the World @ http://www.chemfiesta.com/ Limiting Reagent Problems For the following reactions, find the following: a) Which of the reagents is the limiting reagent? b) What is the maximum amount of each product that can be formed? c) How much of the other reagent is left over after the reaction is complete?
1) Consider the following reaction:
3 NH4NO3 + Na3PO4 (NH4)3PO4 + 3 NaNO3 Answer the questions above, assuming we started with 30.0 grams of ammonium nitrate and 50.0 grams of sodium phosphate.
2) Consider the following reaction:
3 CaCO3 + 2 FePO4 Ca3(PO4)2 + Fe2(CO3)3 Answer the questions at the top of this sheet, assuming we start with 100.0 grams of calcium carbonate and 45.0 grams of iron (II) phosphate .
Adapted from 24 Lessons that Rocked the World @ http://www.chemfiesta.com/ Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Adapted from 24 Lessons that Rocked the World @ http://www.chemfiesta.com/ Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Solutions to the Limiting Reagent Worksheet 1a) ammonium nitrate 1b) 18.6 grams of ammonium phosphate, 31.9 grams of sodium nitrate 1c) 29.5 grams of sodium phosphate 2a) iron (III) phosphate 2b) 46.3 grams of calcium phosphate, 43.8 grams of iron (III) carbonate 2c) 54.0 grams of calcium carbonate
Adapted from 24 Lessons that Rocked the World @ http://www.chemfiesta.com/