UNIT – I (ELECTRO CHEMISTRY)
Electrolytic cell: “A device in which the electrical energy is converted into chemical energy through chemical reaction is called electrolytic cell”. Example: Leclanche cell.
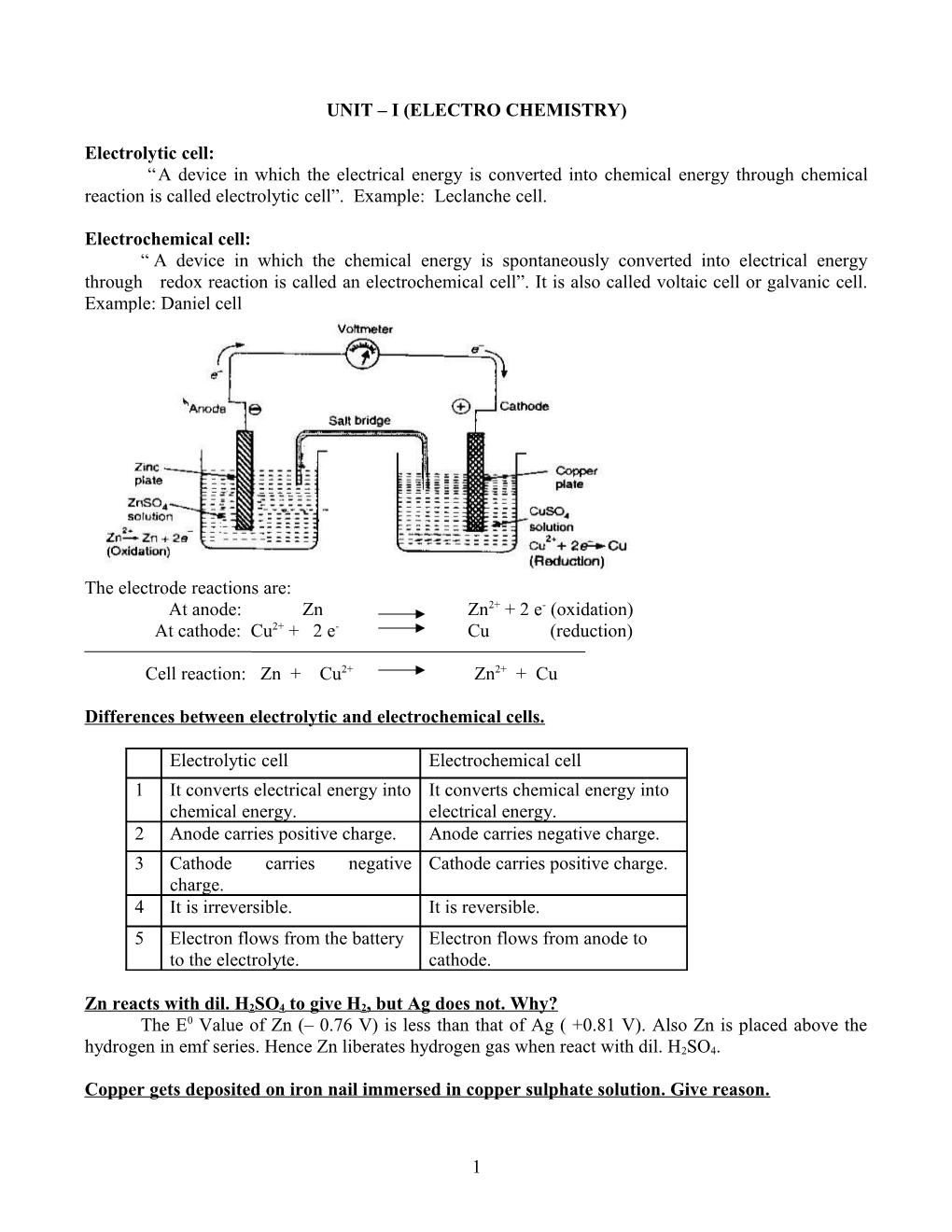
Electrochemical cell: “ A device in which the chemical energy is spontaneously converted into electrical energy through redox reaction is called an electrochemical cell”. It is also called voltaic cell or galvanic cell. Example: Daniel cell
The electrode reactions are: At anode: Zn Zn2+ + 2 e- (oxidation) At cathode: Cu2+ + 2 e- Cu (reduction)
Cell reaction: Zn + Cu2+ Zn2+ + Cu
Differences between electrolytic and electrochemical cells.
Electrolytic cell Electrochemical cell 1 It converts electrical energy into It converts chemical energy into chemical energy. electrical energy. 2 Anode carries positive charge. Anode carries negative charge. 3 Cathode carries negative Cathode carries positive charge. charge. 4 It is irreversible. It is reversible. 5 Electron flows from the battery Electron flows from anode to to the electrolyte. cathode.
Zn reacts with dil. H2SO4 to give H2, but Ag does not. Why? The E0 Value of Zn (– 0.76 V) is less than that of Ag ( +0.81 V). Also Zn is placed above the hydrogen in emf series. Hence Zn liberates hydrogen gas when react with dil. H2SO4.
Copper gets deposited on iron nail immersed in copper sulphate solution. Give reason.
1 The E0 Value of Fe (– 0.44 V) is less than that of Cu (+0.34V), so iron displaces copper from copper sulphate solution. Fe + Cu2+ → Fe2+ + Cu Reversible cells:
Those cells which obey the following three conditions of thermodynamic reversibility are called reversible cells. Eg. Daniel cell.
Thermodynamic conditions:
1. When external emf = 1.1 V, there is no flow of current. 2. When external emf < 1.1 V, there is a current flow in forward direction. 3. When external emf > 1.1 V, there is a current flow in backward direction.
Irreversible cells:
Those cells which do not obey the conditions of thermodynamic reversibility are called irreversible cells. Eg. Dry cell. Similarly Cu and Zn reacts with sulphuric acid, form different set of product shows irreversibility.
Electrode potential:
The tendency of a metallic electrode to gain or lose electrons, when it is in contact with its own salt solution is called electrode potential.
Oxidation potential:
The tendency of a metallic electrode to lose electrons when it is in contact with its own salt solution.
Reduction potential:
The tendency of a metallic electrode to gain electrons when it is in contact with its own salt solution.
Standard electrode potential:
The tendency of a metallic electrode to gain or lose electrons, when it is in contact with its own salt solution of unit molar concentration at 250C.
Salt bridge It is U shaped tube contains saturated solution of KC or NH4NO3 in agar-agar gel. The functions are
1. It maintains electrical neutrality. 2. It eliminates liquid junction potential. 3. It produces connectivity between two half cells. 4. It allows migration of ions from one half cells to another.
2 Nernst equation:
Consider a general reversible redox reaction,
n+ - M (aq) + n e M(s) ……… (1)
Now for a reversible reaction the free energy change ( Δ G) and its equilibrium constant (K) are inter- related as, Δ G = - RT ln K + RT ln {[Products] / [Reactants]} (2)
We know that Δ G0 = - RT ln K (3) and Apply the equation (3) in (2), We get
Δ G = Δ G0+ RT ln {[Products] / [Reactants]} …… (3)
Where Δ G0 is the standard free energy change. The equation (3) is known as Van’t Hoff reaction isotherm.
In a reversible reaction, the electrical energy is produced is the same as decrease in free energy. i.e. – Δ G = nFE and -- Δ G0 = nFEo …. (4)
Where, E = electrode potential, E0 = standard electrode potential and F = Faraday (96,500 Coulomb). Apply the equations (1) and (4) in equation (3), we get
-nFE = -nFE0 + RT ln {[M] / [Mn+]}
Since the concentration of metal, M is unity, we have
-nFE = -nFE0 + RT ln {1 / [Mn+]}
-nFE = -nFE0 - RT ln [Mn+]
Divide this equation by –nF, we get
E = E0 + (RT / nF) ln [Mn+]
E = E0 + (2.303 RT / nF) log [Mn+] …. (5)
This equation (5) is known as Nernst’s equation for electrode potential.
At 250C, E = E0 + (0.0592/ n) log [Mn+]
Similarly Oxidation potential-Nernst Equation
E = E0 - (0.0592/ n) log [Mn+] E – Electrode potential T – 298 K Eo – Standard electrode potential n – No of electrons involved R – Gas constant F – 96500 coulomb
3 Nernst equation for Daniel cell. [Zn2+] [Cu] E = E0 - (2.303 RT / nF) log ------[Cu2+] [Zn] Significance of Nernst equation: 1. If [Mn+] increases, the Ecell is also increases and vice versa. 2. If T increases, the Ecell is also increases and vice versa.
Applications: 1. It is used to study the effect of electrolyte concentration. 2. It is used for the calculation of Ecell. 3. It is used to calculate pH of a solution. 4. It is used for finding the valency of an ion.
EMF:
“The difference of potential which causes flow of current from one electrode which is at higher potential to another electrode which is at lower potential” is called EMF and is expressed in volts. i.e. ECell = ERHE – ELHE
Measurement of emf of a cell:
The emf of a cell can be measured accurately by potentiometric method, which is based on Poggendroff’s compensation principle.
It consists of a uniform wire AB of high resistance. A storage battery of constant emf is connected at the ends A and B of the wire.
The unknown cell X is connected in the circuit.
The end A at which the battery connected and then through a galvanometer G to the Sliding contact D.
The sliding contact is moved along the wire AB till there is no flow of current through the galvanometer. The position of D is then noted. The Ex is proportional to AD.
The cell X is now replaced by the standard cell S of Es is known. As like the above method the AD’ is calculated. The Es is proportional to AD’.
The emf of the unknown cell can be calculated by using the formula, Ex = (AD/AD’) x Es
4 Reference electrodes:
Electrodes of known potential, with which we can compare the potentials of another electrode, are called reference electrodes. Example. S.H.E, calomel electrode, Ag – AgCl electrode, Glass electrode, Quinhydrone electrode, etc.
Standard Hydrogen Electrode:
It is the best primary reference electrode (since Eo=0) It consists of a small platinised Pt foil, which is sealed through the end of a glass tube. This tube is surrounded by another tube, which is sealed to the inner glass tube at the top. The outer glass tube is provided with a side – arm for passing hydrogen gas into the in- between space.The bottom of the outer tube is opened into a bell, around the Pt electrode. Openings in the bell allow the escape of hydrogen gas. The Pt foil is coated with a layer of finely divided Pt, which adsorbs the hydrogen gas and it also speeds up the equilibrium between hydrogen gas and the H+ ions.
This electrode, when dipped in 1N – HCl and when 1 atm H2 passed to give SHE. + Anode: H2 2 H + 2 e + Cathode: 2 H + 2 e H2
Merits: It is used to find the pH value of an unknown solution.
Limitations: It cannot be used in the presence of ions of many metals. It cannot be used in solutions containing redox systems. It is difficult to set up It cannot be used in redox systems It is affected by compounds of Hg, As, S, and oxidizing agents like Fe3+,permanganate, dichromate, etc. It cannot be used in the presence of ions of many metals.
Glass electrode:
It is also called internal reference electrode or indicator electrode. It is highly sensitive to [H+]. It is mostly used in pH measurements.
5 Construction: It consists of a long glass tube with thin walled bulb at the bottom which has low melting point and high electrical conductivity. It contains a solution of 0.1 M HCl. A Pt wire is inserted inside the tube.
It is represented as Pt, HCl (0.1 M) / Glass
Determination of pH of solution: The pH of an unknown solution is found out by coupling the glass electrode with the calomel electrode. The complete cell representation is,
+ Pt, HCl (0.1 M) / Glass / H // KCl(Satd), Hg2Cl(2)(s)/Hg .
The pH of the unknown solution is calculated using the formula
0 pH = ( 0.2422 - E G - ECell ) / 0.0591
Advantages: 1. It is the most convenient and simple to use. 2. Equilibrium is rapidly achieved. 3. The results are accurate. 4. It is not easily poisoned.
Limitations: 1. It can be used in solutions with pH range of 0 to 10. 2. It requires special electronic potentiometers. Glass electrode cannot be used for solution of pH above 9.0? At pH above 9.0, the ions of the solution affect the glass interface and render the electrode useless.
Calomel electrode:
It is the secondary reference electrode. It is the Mercury – Mercurous chloride electrode.
It contain a layer of mercury at the bottom, over which a paste of Hg + Hg2Cl2. The remaining part is filled with a saturated solution of KCl. A Pt wire, dipping into the Hg layer, is responds electrical contact.
6 The side – tube is used for making electrical contact with a salt bridge. The potential of calomel electrode is inversely varies with concentration of KCl.
0 It can be represented as Hg2Cl2(s), KCl(Satd solution). E = 0.2422 V
- Anode reaction: 2 Hg(l) + 2 Cl Hg2Cl(2)(s) + 2 e
- Cathode reaction: Hg2Cl(2)(s) + 2 e Hg(l) + 2 Cl (aq)
Merits: It is simple to construct. It gives accurate results and do not vary with T.
Ion selective electrodes:
The electrodes which have the ability to respond to certain specific ions present in a mixture while ignoring other ions and develop a potential are called ion – selective electrodes.
Applications of ion selective electrodes.
1. Used to determine the concentrations of a number of cations like H+, Li+, Na+, K+, Ag+, Pb2+, etc. 2. Used to determine the concentrations of anions like fluoride, nitrate, cyanide, sulphide and other halide ions, etc. 3. Used for determining the pH of the solution.
EMF (electrochemical) series:
A series in which various electrodes are arranged in the increasing order of their standard reduction potential values based on Hydrogen scale (Eo=0) is called electrochemical series.
Relationship between spontaneity and electrode potential of a cell. 0 a. If E Cell is positive, the cell reaction is spontaneous. 0 b. If E Cell is negative, the cell reaction is non-spontaneous
Potentiometric titrations:
Principle: This method is based on the change in potential of the solution at the end point during titration. In this titration, change in active ion concentration leads to the change in electrode potential.
7 The potential of the indicator electrode (Pt electrode) can be measured using potentiometer by connecting it with a reference electrode (Saturated calomel electrode). The Nernst equation is E = E0 + (RT/nF) logC
(a) Volume of titrant vs EMF (b) Volume of titrant vs(Δ E / ΔV)
The change in potential is noted for each addition of the titrant. The change in potential is small at initial, but it is large at the end point. The end point of the titration is obtained from the plot of volume of titrant vs Δ E / ΔV against the volume of titrant added. The maxima of the curve give the end point.
Potentiometric redox titrations:
Burette solution: K2Cr2O7 solution Pipette solution: unknown 20 ml of FeSO4 + 20 ml of dil. H2SO4 Electrodes used: Pt electrode & calomel electrode. 3+ 2+ Cell representation: Hg / Hg2Cl(2)(s) , KCl // Fe / Fe , Pt
+6 +3 2+ +3 When FeSO4 solution is titrated against K2Cr2O7 solution, Cr reduced to Cr and Fe oxidized to Fe and the change in potential is noted. A graph is drawn by taking volume of K2Cr2O7 in the X axis and change in potential in the Y axis, from which volume of K2Cr2O7 is obtained. From the end point concentration of FeSO4 is calculated.
Potentiometric precipitation titration: Burette solution: KCl solution Pipette solution: 20 ml of unknown AgNO3 solution + 100 ml distilled water Electrodes used: Ag electrode & calomel electrode.
When AgNO3 solution is titrated against KCl solution, the following precipitation reaction takes place. AgNO3 + KCl KNO3 + AgCl
The concentration of Ag+ ion decreases due to the formation of insoluble AgCl and the change in potential is noted. A graph is drawn by taking volume of KCl in X axis and and change in potential in the Y axis , from which volume of KCl is obtained. From the end point the concentration of AgNO3 is calculated.
Conductometric titration of HCl vs NaOH:
8 Principle: This method is based on the change in conductance of the solution during titration. The conductance of the solution depends on the number of free ions, charge on the free ions and mobility of the ions.
Conductometric acid-base titration:
Burette solution: NaOH solution Pipette solution: unknown HCl solution + 100 ml distilled water Electrodes used: Pt electrode & conductivity cell.
When HCl solution is titrated against NaOH solution, the following neutralization reaction occurs.
NaOH + HCl NaCl + H2O
The concentration of fast moving H+ ions of acid neutralized by OH – ions of base to form water. The conductance first decreases and then increases after the end point. A graph is drawn by taking volume of NaOH in X axis and conductance in Y axis, from which volume of NaOH is obtained. From the end point the concentration of HCl is calculated.
Conductance
Volume of NaOH Advantages: 1. It gives more accurate end points. 2. It requires no indicators. 3. It is very useful in case of coloured solution. 4. It is very useful for dilute solutions. 5. It is very useful for titrating weak acid’s against weak base’s. 6. In this method, no keen observance is near the end point, since it is detected graphically.
QUESTION BANK Part – A 1. What is electrode potential? 2. What is an electrochemical cell? 3. Define reduction potential? 4. What are reference electrodes? Give an example. 5. Why is salt bridge used in the construction of a cell? 6. Why glass electrode cannot be used for solution of pH above 9.0 ? 7. Write the mathematical expression for Nernst’s equation for a Daniel cell.
8. Why we use NH4NO3 or KCl for preparing salt bridges? 9. Differentiate between electrolytic cell and an electrochemical cell. 10. Give examples of reference electrodes. 11. Give Nernst equation. 12. Define single electrode potential.
9 13. What are reversible cells ? Give an example. 14. What are irreversible cells? Give an example.
15. Zn reacts with dil. H2SO4 to give H2, but Ag does not. Why? 16. How is electrode potential developed? 17. Define Standard electrode potential. 18. What is electrochemical series? 19. What is an ion selective electrode? 20. What is the effect of electrolyte concentration on electrode potential?
21. A Zn rod is placed in 0.1 M ZnSO4 solution at 298 K. Write the electrode reactions and Calculate the potential 0 of the electrode. E Zn = -0.76 V. 22. Write the Nernst equation for potentiometry ofor Fe2+ / Fe3+ system. 23. Write advantages of conductometric titration over an ordinary volumetric analysis. 24. How is the free energy change for the cell reaction is related to the cell emf.?
Part – B 1. Derive Nernst equation for single electrode potential and explain the terms involved in it. Write its applications. 2. Explain the construction and functioning of a Daniel cell. 3. Explain the measurement of pH of a solution using glass electrode. Mention the advantages of this electrode. 4. Describe the following electrodes giving the diagram, electrode notation and electrode reaction. (i) Standard hydrogen electrode (ii) Calomel electrode. 5. Define electromotive force. How is it measured by potentiometric method? 6. What is electrochemical series? Give its applications with suitable examples. 7. Explain NaOH – HCl titration conductometrically.
8. Explain the principle of potentiometric titration of K2Cr2O7 Vs FeSO4 . 9. Discuss the potentiometric precipitation titration with an example.
10. Calculate the emf of a Daniel cell at 298 K, when the concentration of ZnSO4 and CuSO4 are 0.001 M and 0.1 M respectively. The standard electrode potential of cell is 1.1 V. 11. Write the half – cell reactions and net cell reactions of the following cell: Zn / Zn2+(1M)// Cu2+(1M) / Cu. Find the emf of the above cell, given that the SRP of Zn and Cu are -0.76 V and +0.34 V. 12. What is the concentration of Ni2+ in the cell at 250C, if the emf is 0.601 V? Ni / Ni2+ ( ? )// Cu2+(0.75 M) / Cu . Given that Standard reduction potentials of Ni and Cu are 0.25 V and 0.34 V respectively. 13. Calculate the standard free energy change of the following cell reaction: Fe2+ + Ag+ → Fe3+ +Ag Given that E0 Fe3+ / Fe2+ = 0.77 V and E0 Ag+ / Ag = 0.80 V. 14. Write the half cell reactions and net cell reactions for the cell: Cd / Cd2+( 0.01M )// Cu2+(0.5 M) / Cu. The SRP of Cd and Cu are -0.4 V & 0.34 V respectively. Calculate the emf of the cell. 15. Calculate the standard electrode potential of Cu2+ / Cu, if the electrode potential at 298 K is 0.296 V, when the concentration of Cu2+ is 0.015 M.
*************
10