Version: August 9, 2017 iPHIS Case ID #: Ontario Infant Botulism Investigation Tool
for interview with case ♦ System-Mandatory Required Personal Health Legen Information d
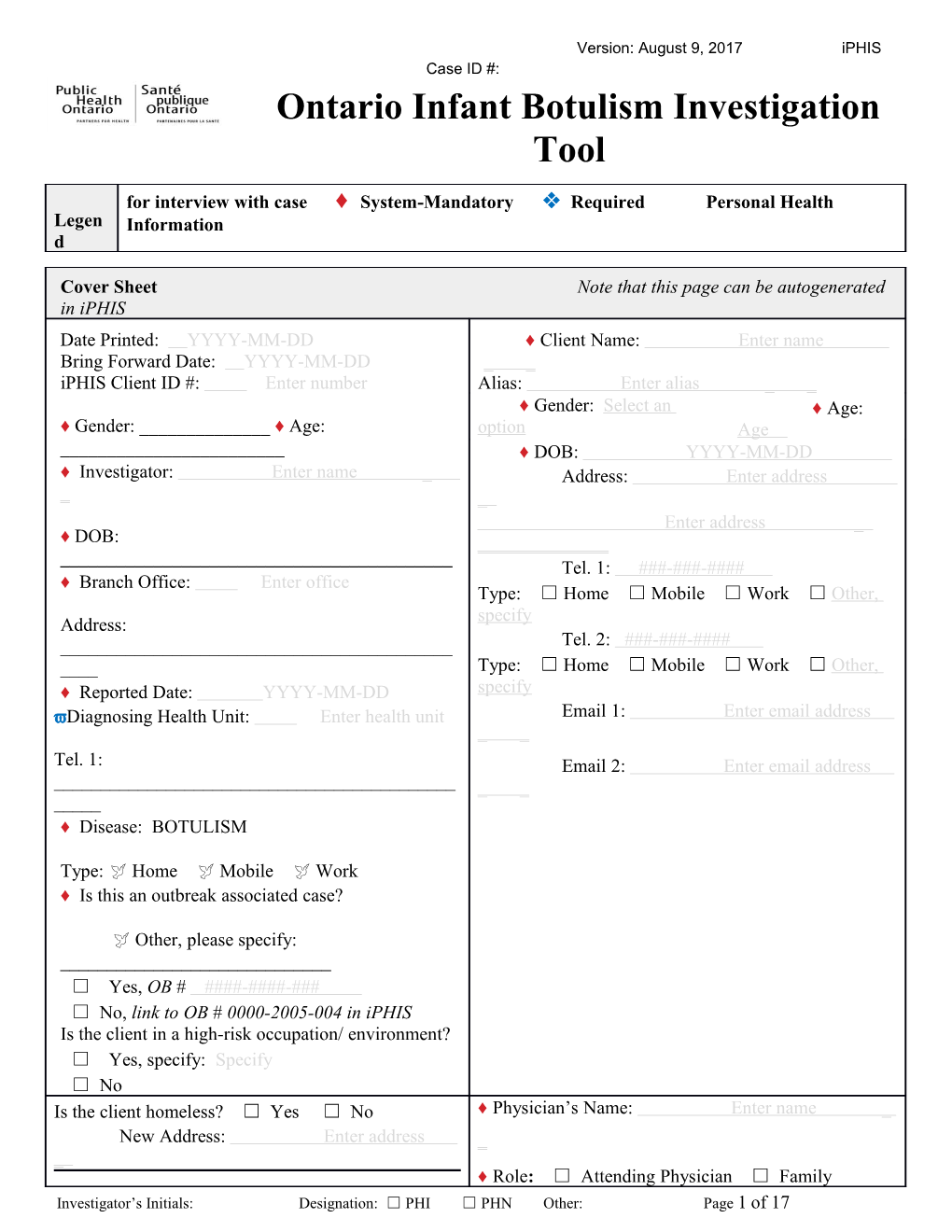
Cover Sheet Note that this page can be autogenerated in iPHIS Date Printed: YYYY-MM-DD ♦ Client Name: Enter name Bring Forward Date: YYYY-MM-DD _ _ iPHIS Client ID #: Enter number Alias: Enter alias _ _ ♦ Gender: Select an ♦ Age: ♦ Gender: ______♦ Age: option Age ______♦ DOB: YYYY-MM-DD ♦ Investigator: Enter name _ Address: Enter address _ _ Enter address _ ♦ DOB: ______Tel. 1: ###-###-#### ♦ Branch Office: Enter office Type: ☐ Home ☐ Mobile ☐ Work ☐ Other, specify Address: ______Tel. 2: ###-###-#### ____ Type: ☐ Home ☐ Mobile ☐ Work ☐ Other, ♦ Reported Date: YYYY-MM-DD specify Diagnosing Health Unit: Enter health unit Email 1: Enter email address _ _ Tel. 1: Email 2: Enter email address ______♦ Disease: BOTULISM
Type: Home Mobile Work ♦ Is this an outbreak associated case?
Other, please specify: ______☐ Yes, OB # ####-####-### ☐ No, link to OB # 0000-2005-004 in iPHIS Is the client in a high-risk occupation/ environment? ☐ Yes, specify: Specify ☐ No Is the client homeless? ☐ Yes ☐ No ♦ Physician’s Name: Enter name _ New Address: Enter address _ _ ♦ Role: ☐ Attending Physician ☐ Family Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 1 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
♦ Language: Specify _ _ Physician Translation required? ☐ Yes ☐ No ☐ Specialist ☐ Proxy respondent Walk-In Physician Name: Enter name _ ☐ Other ☐ _ Unknown ☐ Parent/Guardian ☐ Spouse/Partner OPTIONAL ☐ Other Specify _ _ Additional Physician’s Name: Enter name _ Address: Enter address _ Tel: ###-###-#### Fax: ###-###-#### Role: Enter role _ _
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 2 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Verification of Client’s Identity & Notice of Collection Client’s identity verified? ☐ Yes, specify: ☐ DOB ☐ Postal Code ☐ Physician ☐ No Notice of Collection Please consult with local privacy and legal counsel about PHU-specific Notice of Collection requirements under PHIPA s. 16. Insert Notice of Collection, as necessary.
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 3 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Record of File ♦ Responsible Date ♦ Investigator Investigator Designation Health Unit Investigator’s ’s Signature ’s Initials Name Investigation Start ☐ PHI ☐ Date PHN
☐ Other ______Assignment Date ☐ PHI ☐ PHN
☐ Other ______
Call Log Details Date Start Time Type Call To/From Outcome Investigator’ of (contact made, v/m, text, s initials Call email, no answer, etc.) Call ☐ 1 Outgoi ng
☐ Incomi ng Call ☐ 2 Outgoi ng
☐ Incomi ng Call ☐ 3 Outgoi ng
☐ Incomi ng Call ☐ 4 Outgoi ng
☐ Incomi ng Call ☐ 5 Outgoi ng
☐ Incomi ng Call ☐ 6 Outgoi ng ☐
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 4 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Incomi ng Date letter sent:
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 5 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Case Details ♦ Aetiologic Agent Clostridium Botulinum ☐ Toxin A ☐ Toxin E Further ☐ Toxin B ☐ Toxin F Differentiation ☐ Spore A ☐ Spore E Enter this Subtype ☐ Infant ☐ Spore B ☐ Spore F selection in the ☐ Other ‘free text’ field in iPHIS ☐ Unspecified ☐ Confirmed ☐ Person Under Investigation ♦ Classification YYYY-MM- ♦ Classification ☐ Probable ☐ Does Not Meet Definition Date DD Do not close case as PUI
☐ Confirmed ☐ Person Under Investigation ♦ Outbreak ♦ Outbreak Case YYYY-MM- ☐ Probable ☐ Does Not Meet Definition Classification Classification DD Do not close case as PUI Date ☐ Complete ☐ Closed- Duplicate-Do Not Use YYYY-MM- Disposition ☐ ☐ Disposition Date ♦ Entered In Error Lost to Follow ♦ DD Up ☐ Does Not Meet Definition ☐ Untraceable YYYY-MM- ☐ Status Date Closed ♦ DD ☐ Open (re- YYYY-MM- ♦ Status ♦ Status Date opened) DD YYYY-MM- ☐ Status Date Closed ♦ DD ☐ Medium ☐ (At health unit’s ♦ Priority ☐ High Low discretion) Lab specimens Specimen Type Collection Date Result Date Result Comments e.g., blood, stool, gastric aspirate, food YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD YYYY-MM-DD
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 6 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Symptoms The incubation period of infant botulism is unknown since the time of spore germination, growth and toxin production is unknown. It is suggested that investigations focus on the month prior to onset of illness in an attempt to investigate the source of botulism. ♦ ♦ Use Onset Recovery Date Sympt Respons as Onset Time YYYY-MM-DD oms e Onset Date 24-HR (choose one) (choose YYYY- Clock one) MM- HH:MM DD (discreti onary)
Don’t Not Yes No Refused Know Asked
Anorexi ☐ ☐ ☐ ☐ ☐ ☐ YYYY- YYYY- YYYY- a [loss of MM-DD MM-DD MM-DD appetite] Constipa ☐ ☐ ☐ ☐ ☐ ☐ YYYY- YYYY- YYYY- tion MM-DD MM-DD MM-DD Eyelid(s) ☐ ☐ ☐ ☐ ☐ ☐ YYYY- YYYY- YYYY- , MM-DD MM-DD MM-DD drooping Infant, ☐ ☐ ☐ ☐ ☐ ☐ YYYY- YYYY- YYYY- loss of MM-DD MM-DD MM-DD head control Infant, ☐ ☐ ☐ ☐ ☐ ☐ YYYY- YYYY- YYYY- poor MM-DD MM-DD MM-DD muscle tone Infant, weak cry ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- MM-DD DD DD Lethargy ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- MM-DD DD DD Paralysis ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- MM-DD DD DD Respiratory ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- Failure MM-DD DD DD Swallowing ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- difficulty MM-DD DD DD [dysphagia] Weak ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- MM-DD DD DD Other (specify) ☐ ☐ ☐ ☐ ☐ YYYY- YYYY-MM- YYYY-MM- Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 7 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
MM-DD DD DD
Note: This list is not comprehensive. There are additional symptoms listed in iPHIS.
♦ Complications ☐ None ☐ Other ☐ Unknown
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 8 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Hospitalization & Treatment Mandatory in iPHIS only if admitted to hospital Did you bring your child ☐ Yes If yes, Name of hospital: to an emergency room? Date(s): YYYY-MM-DD ☐ No ♦ Was your child admitted ☐ Yes If yes, Name of hospital: to hospital as a result of ♦ Date of admission: ☐ No their illness (not including Date of discharge: stay in the emergency ☐ Don’t room)? recall ☐ client remains in hospital ☐ Unknown discharge date
→ For iPHIS data entry – if the case is hospitalized, enter information under Cases > Case > Interventions. Was antitoxin ☐ Yes If yes, date given: administered? ☐ No ☐ Don’t know Treatment of infant botulism requires Botulism Immune Globulin, Intravenous (BIG-IV) or BabyBIG ®. This can only be obtained through Health Canada’s Special Access Program (SAP). For more information on placing a request for BabyBIG ®refer to the Botulism – Guide for Healthcare Professionals Treatment information can be entered in iPHIS under Cases > Case > Rx/Treatments>Treatment as per current iPHIS User Guide
Date of Onset, Age and Gender Complete this section if submission of pages 5-8 to Public Health Ontario is required Date of Onset: Age: Age Gender: Select an option
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 9 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Preliminary Questions Response Details U n Y N s e o u s r e Do you have any idea how your child ☐ ☐ ☐ became sick? Was your child on any specific diet(s) ☐ ☐ ☐ in the 3 days prior to the onset of their illness (e.g. vegetarian, vegan, gluten- free, kosher, halal, etc.)? Did you attend any special functions ☐ ☐ ☐ with your child such as weddings, parties, showers, family gatherings or group meals in the 3 days prior to the onset of your child’s illness?
Food History In the four weeks before your child became ill, can you list all of the food items that you would routinely or typically provide your child on any given day? Could you also list all of the food items that you fed your child in the month before your child became ill that you do not typically or routinely feed your child?
Day Food consumed
Typical Day
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 10 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Non-routine food items
Behavioural Response Details Social Risk iPHIS character limit: 50. Factors Yes No Unknown Not asked
Commercial ☐ ☐ ☐ ☐ jarred baby food
Consumption ☐ ☐ ☐ ☐ of honey (Specify unpasteurized vs. pasteurized) Contact or ☐ ☐ ☐ ☐ exposure to soil/ dust Home ☐ ☐ ☐ ☐ renovations Lives near ☐ ☐ ☐ ☐ construction site Close proximity ☐ ☐ ☐ ☐ to farms where soil is being disturbed (house is dusty from land being Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 11 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #: disturbed)
☐ ☐ ☐ ☐ Cookies/biscuits Dry infant ☐ ☐ ☐ ☐ cereal Home ☐ ☐ ☐ ☐ prepared baby food Other ☐ ☐ ☐ ☐ (specify) Solid food ☐ ☐ ☐ ☐ introduction in past 30 days Travel ☐ ☐ ☐ ☐ outside province in the 30 days prior to illness onset (specify) Within Canada ☐ ☐ ☐ ☐ From: To: Where:
Outside of ☐ ☐ ☐ ☐ From: Canada To: Where: Hotel/Resort:
Behavioural Response Details Social Risk iPHIS character limit: 50. Please use ‘Notes’ section if Factors Yes N needed o
Unknown ☐ ☐ → For iPHIS data entry – check Yes for Unknown if all other Behavioural Risk Factors are No or Unknown ♦ Create Exposures Identify Exposures to be entered in iPHIS. → For iPHIS data entry – record details of exposure(s) in iPHIS Case Exposure Form as required.
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 12 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Premises Referral Has a food premises been identified as a ☐ Yes If yes, refer premises to the Food Safety Program and possible source? create an exposure as appropriate. ☐ No
Education/Counselling Discuss the relevant sections with case ☐ Do not feed honey to infants less than one year of age
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 13 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Outcome Mandatory in iPHIS only if Outcome is Fatal Outcome ☐ Unknown ☐ ♦ Fatal ♦ Cause(s) of Death? If fatal, ☐ Ill ☐ complete Pending disposition type ☐ Residual effects ☐ and facility name Recovered in iPHIS If fatal, complete section below under Outcome
♦ Type of ☐ Reportable Disease Contributed to but was Not the underlying cause of death Death ☐ Reportable Disease was the Underlying cause of Death ☐ Reportable Disease was Unrelated to the cause of Death Unknown ☐ Outcome Date ☐ Yes Specify source (e.g., death certificate) Date YYYY-MM-DD Accurat ☐ e No
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 14 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Thank you
Thank you for your time. This information will be used to help prevent future cases of infant botulism.
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 15 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Interventions Intervention Type Intervention Investigator’s ♦ Start Date End Date implemented initials YYYY-MM-DD YYYY-MM-DD (check all that apply) Counselling ☐ YYYY-MM-DD YYYY-MM-DD Education ☐ YYYY-MM-DD YYYY-MM-DD (e.g. disease fact sheet, general food safety chart/cooking temperature chart, hand washing information) ER visit ☐ YYYY-MM-DD YYYY-MM-DD Exclusion ☐ YYYY-MM-DD YYYY-MM-DD Food Recall ☐ YYYY-MM-DD YYYY-MM-DD Hospitalization ☐ YYYY-MM-DD YYYY-MM-DD Letter - Client ☐ YYYY-MM-DD YYYY-MM-DD Letter - Physician ☐ YYYY-MM-DD YYYY-MM-DD Other (i.e., contacts assessed, ☐ YYYY-MM-DD YYYY-MM-DD PHI/PHN contact information) → For iPHIS data entry – enter information under Cases > Case > Interventions.
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 16 of 17 Ontario Infant Botulism Investigation Tool Version: August 9, 2017 iPHIS Case ID #:
Progress Notes
If you have any comments or feedback regarding this Investigation Tool, please email us at [email protected].
Investigator’s Initials: Designation: ☐ PHI ☐ PHN Other: Page 17 of 17