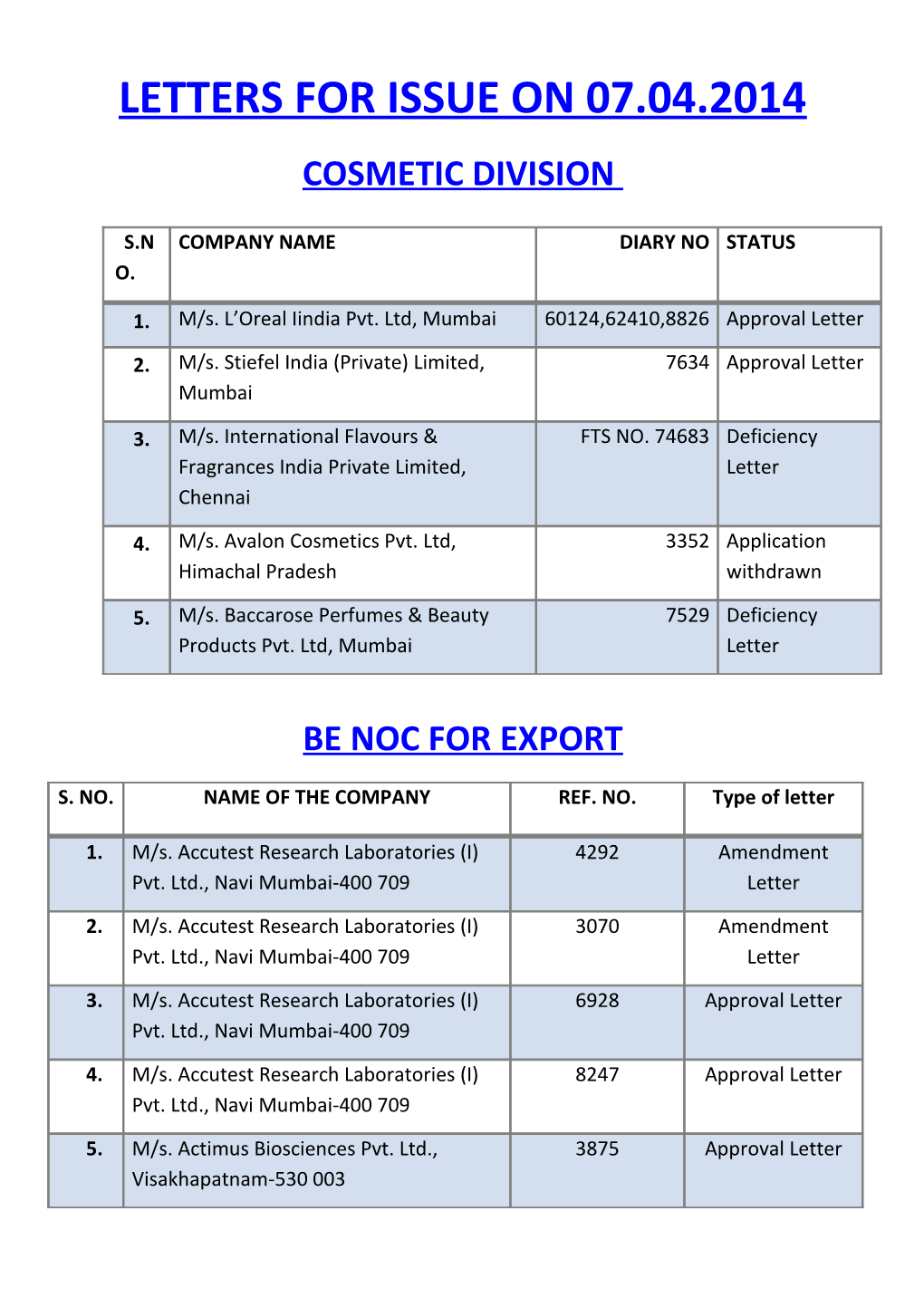
LETTERS FOR ISSUE ON 07.04.2014 COSMETIC DIVISION
S.N COMPANY NAME DIARY NO STATUS O.
1. M/s. L’Oreal Iindia Pvt. Ltd, Mumbai 60124,62410,8826 Approval Letter
2. M/s. Stiefel India (Private) Limited, 7634 Approval Letter Mumbai
3. M/s. International Flavours & FTS NO. 74683 Deficiency Fragrances India Private Limited, Letter Chennai
4. M/s. Avalon Cosmetics Pvt. Ltd, 3352 Application Himachal Pradesh withdrawn
5. M/s. Baccarose Perfumes & Beauty 7529 Deficiency Products Pvt. Ltd, Mumbai Letter
BE NOC FOR EXPORT
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Accutest Research Laboratories (I) 4292 Amendment Pvt. Ltd., Navi Mumbai-400 709 Letter
2. M/s. Accutest Research Laboratories (I) 3070 Amendment Pvt. Ltd., Navi Mumbai-400 709 Letter
3. M/s. Accutest Research Laboratories (I) 6928 Approval Letter Pvt. Ltd., Navi Mumbai-400 709
4. M/s. Accutest Research Laboratories (I) 8247 Approval Letter Pvt. Ltd., Navi Mumbai-400 709
5. M/s. Actimus Biosciences Pvt. Ltd., 3875 Approval Letter Visakhapatnam-530 003 6. M/s. Aizant Drug Research Solutions Pvt. 6549 Approval Letter Ltd., Hyderabad-500 010
7. M/s. Alembic Pharmaceuticals Ltd., 14136 Amendment Vadodara-390 003 Letter
8. M/s. Apotex Research Pvt. Ltd., 3917 Clarification Bangalore-560 099 Letter
9. M/s. Apotex Research Pvt. Ltd., 2995 Amendment Bangalore-560 099 Letter
10. M/s. Axis Clinicals Ltd., Hyderabad-500 825 Clarification 049 Letter
11. M/s. Azidus Laboratories Ltd., Chennai- 31901 Approval Letter 600 048
12. M/s. Cliantha Research Limited, 6222 Approval Letter Ahmedabad-380 054
13. M/s. Cliantha Research Limited, 5005 Approval Letter Ahmedabad-380 054
14. M/s. Dr. Reddy’s Laboratories Ltd., 2216 Clarification Hyderabad-500 016 Letter
15. M/s. Drug Monitoring Research Institute 10217 Approval Letter (P) Ltd., Navi Mumbai-400 701
16. M/s. Emcure Pharmaceuticals Limited, 2133 Amendment Pune-411 026 Letter
17. M/s. Emcure Pharmaceuticals Limited, 3557 Approval Letter Pune-411 026
18. M/s. GVK Biosciences Pvt. Ltd., 3426 Amendment Ahmedabad-380 051 Letter
19. M/s. Jubilant Life Sciences Limited, 3635 Approval Letter Noida-201 307, UP
20. M/s. Lambda Therapeutic Research Ltd., 1282 Clarification Ahmedabad-380 061 Letter
21. M/s. Lambda Therapeutic Research Ltd., 3311 Amendment Ahmedabad-380 061 Letter
22. M/s. Lotus Labs Pvt. Ltd., Bangalore-560 2146 Amendment 052 Letter
23. M/s. Lupin Bioresearch Center, Pune-411 3876 Approval Letter 021
24. M/s. Manipal AcuNova Limited, Manipal 6227 Approval Letter -576 104
25. M/s. Manipal Acunova Ltd., Mangalore- 1037 Amendment 575 001 Letter
26. M/s. Norwich Clinical Services Pvt Ltd, 6702 Approval Letter Bangalore-560034
27. M/s. Ranbaxy Laboratories Ltd., Gurgaon- 11929 Amendment 122 015 Letter
28. M/s. Ranbaxy Laboratories Ltd., New 6813 Approval Letter Delhi-110 019
29. M/s. Reliance Life Sciences Pvt. Ltd., Navi 49953 Rejection Letter Mumbai-400 701
30. M/s. Reliance Life Sciences Pvt. Ltd., Navi 62687 Approval Letter Mumbai-400 701
31. M/s. Reliance Life Sciences Pvt. Ltd., Navi 11115 Approval Letter Mumbai-400 701
32. M/s. Synchron Research Services Pvt. 6460 Approval Letter Ltd., Ahmedabad-380 054
33. M/s. Themis Medicare Ltd., Mumbai-400 64709 Rejection Letter 104
34. M/s. Themis Medicare Ltd., Mumbai-400 64708 Rejection Letter 104
35. M/s. Torrent Pharmaceuticals Ltd. 712 Amendment (Research Centre), Gujarat Letter
36. M/s. Torrent Pharmaceuticals Ltd. 52383 Amendment (Research Centre), Gujarat Letter 37. M/s. Veeda Clinical Research Pvt. Ltd., 8739 Approval Letter Ahmedabad-380 015
38. M/s. Veeda Clinical Research Pvt. Ltd., 6637 Approval Letter Ahmedabad-380 015
39. M/s. Veeda Clinical Research Pvt. Ltd., 8740 Approval Letter Ahmedabad-380 015
40. M/s. Veeda Clinical Research Pvt. Ltd., 6590 Approval Letter Ahmedabad-380 015
41. M/s. Veeda Clinical Research Pvt. Ltd., 5771 Approval Letter Ahmedabad-380 015
42. M/s. Vimta Labs Ltd., Hyderabad-500 051 4611 Clarification Letter
43. M/s. Watson Pharma Pvt. Ltd., Navi 1021 Clarification Mumbai-400 614 Letter
44. M/s. Wockhardt Limited - CPB, 3521 Amendment Aurangabad-431 210 Letter
ONLY TEST LICENCE FOR BE
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Aizant Drug Research Solutions Pvt. 6548 Test Licence Ltd., Hyderabad-500 010
2. M/s. Emcure Pharmaceuticals Limited, 3363 Test Licence Pune-411 026
3. M/s. Kusum Healthcare Pvt. Ltd., New 3420 Test Licence Delhi-110 020
4. M/s. Lotus Labs Pvt. Ltd., Bangalore-560 2255 Test Licence 052
5. M/s. Ranbaxy Laboratories Ltd., 4201 Test Licence Gurgaon-122 015
6. M/s. Ranbaxy Laboratories Ltd., 54279 Test Licence Gurgaon-122 015
7. M/s. Ranbaxy Laboratories Ltd., 54276 Test Licence Gurgaon-122 015
8. M/s. Ranbaxy Laboratories Ltd., 54277 Test Licence Gurgaon-122 015
9. M/s. Ranbaxy Laboratories Ltd., 54278 Test Licence Gurgaon-122 015
10. M/s. Ranbaxy Laboratories Ltd., 7822 Test Licence Gurgaon-122 015
11. M/s. Ranbaxy Laboratories Ltd., 8252 Test Licence Gurgaon-122 015
12. M/s. Reliance Life Sciences Pvt. Ltd., 12860 Test Licence Navi Mumbai-400 701
13. M/s. Veeda Clinical Research Pvt. Ltd., 7865 Test Licence Ahmedabad-380 015
SUBSEQUENT NEW DRUG S. NAME OF THE FIRM REFERENCE FTS NO. TYPE OF LETTER NO. NO.
1. M/s Intas 34364 - Approval Letter Pharmaceuticals (2)
2. M/s MSN Lab Ltd. 52168 - Approval Letter
3. M/s Glaxo Smithkline 32453 Approval Letter
4. M/s Akums Drugs 63648 87805 Deficiency Letter
VACCINE DIVISION
S. No. COMPANY NAME Dy.No. STATUS 1 M/s Kasiak Research Pvt Ltd 28015 Approval letter
2 M/s Lupin Limited 7379 Permission Letter
3 M/s Globion India Pvt Ltd 8204 Approval letter
4 M/s Panacea Biotec Ltd 56138 Approval letter & clarification letter
5 M/s Sanofi Synthelabo (India) 2456 Permission Letter Ltd
6 M/s LG Life Sciences India Pvt 11381 Clarification letter Ltd
7 M/s Jhonson & Jhonson Ltd 9691 Clarification letter
8 M/s Shantha Biotechnics 60841 Clarification letter Limited
9 M/s Human Biological Clarification letter Institute
10 M/s Bharat Biotech Clarification letter International Ltd
11 M/s Wockhardt Ltd Clarification letter
12 M/s Stallen South Asia Pvt, 36443 Approval letter Ltd
13 M/s Intervet India Pvt, Ltd 7937 Approval letter
14 M/s Cadila Pharmaceuticals 9013 (fts no) Approval letter Ltd
BIOLOGICAL DIVISION
S. No. COMPANY NAME Dy.No. STATUS 1 M/s Indian Immunologicals 4141 Approval letter Limited
FDC DIVISION
S.NO. NAME OF COMPANY DIARY DOCUMENT NO 1. M/s. Ajanta Pharma Ltd., 9591 Letter
TEST LICENCES
S. No. Name of the Company Diary No. Type of Letter
1. Abbott Healthcare Pvt Ltd 8615, Approval Letter 13056
2. Actavis Pharma Development 8805, Approval Letter Centre Pvt Ltd 13412
3. Agila Specialties Pvt Ltd 4913, 7510 Approval Letter
4. Agila Specialties Pvt. Ltd 8966, Approval Letter 13821
5. Agila Specialties Pvt. Ltd 8974, Approval Letter 13825
6. Agila Specialties Pvt. Ltd 8973, Approval Letter 13828
7. Alembic Pharmaceuticals Ltd 8955, Approval Letter 13805
8. Alembic Pharmaceuticals Ltd 8954, Approval Letter 13800
9. Alembic Pharmaceuticals Ltd 8953, Approval Letter 13793
10. Alembic Pharmaceuticals Ltd 8959, Approval Letter 13815 11. Alembic Pharmaceuticals Ltd 8958, Approval Letter 13814
12. Alembic Pharmaceuticals Ltd 8957, Approval Letter 13811
13. Alembic Pharmaceuticals Ltd 8956, Approval Letter 13809
14. Alembic Pharmaceuticals Ltd 8960, Approval Letter 13817
15. Alembic Pharmaceuticals Ltd 8965, Approval Letter 13819
16. Alkem Laboratories Ltd 8592, Approval Letter 13111
17. Alphamed Formulations Pvt. 8688, Approval Letter Ltd. 13514
18. APL Research Centre 8624, Approval Letter 13059
19. APL Research Centre 8629, Approval Letter 13083
20. APL Research Centre 8628, Approval Letter 13086
21. APL Research Centre 8627, Approval Letter 13088
22. APL Research Centre 8626, Approval Letter 13092
23. APL Research Centre 8625,1309 Approval Letter 5
24. Apotex Research Pvt Ltd 8867, Approval Letter 13876
25. Biogen Idec Biotech India Pvt 9013, Approval Letter Ltd 13852
26. Cadila Healthcare Ltd 9030, Approval Letter 13875
27. Cadila Healthcare Ltd 9029, Approval Letter 13874
28. Cadila Healthcare Ltd 9020, Approval Letter 13861
29. Cadila Healthcare Ltd 9021, Approval Letter 13863
30. Cadila Healthcare Ltd 9022, Approval Letter 13865
31. Cadila Healthcare Ltd 9026, Approval Letter 13866
32. Cipla Ltd 8801, Approval Letter 13338
33. Cipla Ltd 8800, Approval Letter 13341
34. Cipla Ltd 8798, Approval Letter 13342
35. Cipla Ltd 8799, Approval Letter 13345
36. Cipla Ltd 8797, Approval Letter 13349
37. Cipla Ltd 8796, Approval Letter 13351
38. Cipla Ltd 8795, Approval Letter 13353
39. Cipla Ltd 8794, Approval Letter 13355
40. Claris LifeSciences Ltd 8875, Approval Letter 13878
41. Dr. Reddy’s Laboratories Ltd 8549, Approval Letter 13020 42. Dr. Reddy’s Laboratories Ltd 8550, Approval Letter 13013
43. Dr. Reddy’s Laboratories Ltd 8548, Approval Letter 13031
44. Famy Care Ltd 9016, Approval Letter 13857
45. Famy Care Ltd 2039, Approval Letter 13746
46. Getz Pharma Research Pvt Ltd 8902, Approval Letter 13880
47. Gland Pharma Ltd 3742, Approval Letter 5758, 11933, 18058
48. Glenmark Generics Ltd 8821, Approval Letter 13419
49. Glenmark Generics Ltd 8829, Approval Letter 13419
50. Glenmark Generics Ltd 9025, Approval Letter 13867
51. Glenmark Pharmaceuticals Ltd 8822, Approval Letter 13417
52. Hospira Healthcare India Pvt. 8583, Approval Letter Ltd. 13128
53. Hospira Healthcare India Pvt. 8584, Approval Letter Ltd. 13125
54. Hospira Healthcare India Pvt. 8585, Approval Letter Ltd. 13118
55. Hospira Healthcare India Pvt. 8586, Approval Letter Ltd. 13112
56. Indeus Life Sciences Pvt. Ltd 8538, Approval Letter 13018 57. Indeus Life Sciences Pvt. Ltd. 8539, Approval Letter 13015
58. Ipca Laboratories Limited 8694, Approval Letter 13466
59. Ipca Laboratories Limited 8695, Approval Letter 13501
60. Ipca Laboratories Limited 8696, Approval Letter 13498
61. Ipca Laboratories Limited 8698, Approval Letter 13496
62. Johnson and Johnson Ltd 8693, Approval Letter 13472
63. Johnson and Johnson Ltd 8733, Approval Letter 13439
64. Jubilant Life Sciences Ltd 8850, Approval Letter 13475
65. Lupin Ltd 8756, Approval Letter 13451
66. Mylan Laboratories Ltd 8736, Approval Letter 13444
67. Mylan Laboratories Ltd 8757, Approval Letter 13465
68. Mylan Laboratories Ltd 8758, Approval Letter 13402
69. Mylan Laboratories Ltd 8734, Approval Letter 13431
70. National Institute of Mental 562, 17512 Approval Letter Health & Neuro Sciences
71. National Institute of Mental 560, 17512 Approval Letter Health & Neuro Sciences 72. National Institute of Mental 556, 17512 Approval Letter Health & Neuro Sciences
73. National Institute of Mental 557, 17512 Approval Letter Health & Neuro Sciences
74. Nektar Therapeutics India Pvt 8595, Approval Letter Ltd 13044
75. Nektar Therapeutics India Pvt 8596, Approval Letter Ltd 13046
76. Novartis Healthcare Pvt Ltd 8650, Approval Letter 13065
77. Novartis Healthcare Pvt Ltd 8647, Approval Letter 13067
78. Novartis Healthcare Pvt Ltd 8646, Approval Letter 13069
79. Novartis Healthcare Pvt Ltd 8645, Approval Letter 13071
80. Novartis Healthcare Pvt Ltd 8643, Approval Letter 13079
81. Novartis Healthcare Pvt Ltd 8648, Approval Letter 13099
82. Novartis Healthcare Pvt Ltd 8649, Approval Letter 13101
83. Par Formulations Pvt Ltd 8658, Approval Letter 13037
84. Perrigo Laboratories India Pvt 8996, Approval Letter Ltd 13832
85. Perrigo Laboratories India Pvt 8997, Approval Letter Ltd 13836
86. Perrigo Laboratories India Pvt 8998, Approval Letter Ltd 13838 87. Perrigo Laboratories India Pvt 9001, Approval Letter Ltd 13840
88. Piramal Enterprises Ltd 8945, Approval Letter 13791
89. Piramal Enterprises Ltd 8944, Approval Letter 13788
90. Sanofi-Synthelabo (India) Ltd. 8972, Approval Letter 13824
91. Sanofi-Synthelabo (India) Ltd. 8971, Approval Letter 13822
92. Sentiss Pharma Ltd 3390, Approval Letter 5459, 8869, 13882
93. Sidmak Laboratories Ltd 2944, Approval Letter 4839, 9988, 15324,
94. Tevapharm India Pvt Ltd 8793, Approval Letter 13359
95. Tevapharm India Pvt Ltd 8792, Approval Letter 13395
96. USV Ltd 9009, Approval Letter 13844
97. USV Ltd 9011, Approval Letter 13847
98. Vimta Labs Ltd 8738, Approval Letter 13455
99. Vimta Labs Ltd 8737, Approval Letter 13462
100. Vimta Labs Ltd 8732, Approval Letter 13435 101. Watson Pharma Pvt. Ltd 8724, Approval Letter 13510
102. Watson Pharma Pvt. Ltd 8723, Approval Letter 13506
103. Wockhardt Ltd 65116, Approval Letter 89225
104. Wockhardt Ltd 8935, Approval Letter 13749
105. Wockhardt Ltd 8934, Approval Letter 13742
NEW DRUG
S.NO. NAME OF APPLICANT DIARY NO. TYPE OF LETTER 1. M/s. Y.R. Gaitonde Medical - Approval Letter (2) Education and Research Foundation 2. M/s. Intas Pharmaceuticals - Clarification Letter