CHM 201 EXAM 1_Example Students are responsible for all material from the notes and textbook. There will also be a set of multiple choice questions.
Question I
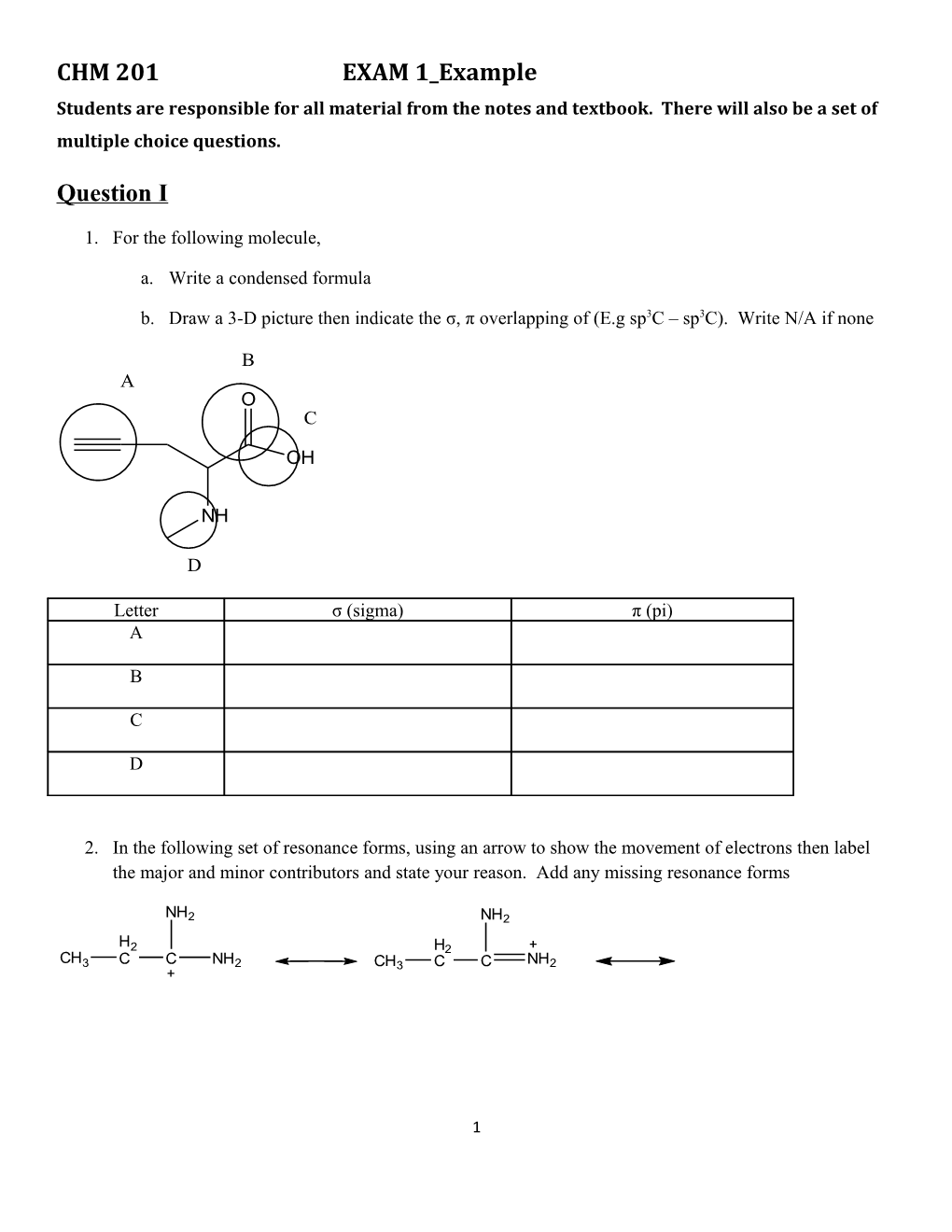
1. For the following molecule,
a. Write a condensed formula
b. Draw a 3-D picture then indicate the σ, π overlapping of (E.g sp3C – sp3C). Write N/A if none
B A O C
OH
NH
D
Letter σ (sigma) π (pi) A
B
C
D
2. In the following set of resonance forms, using an arrow to show the movement of electrons then label the major and minor contributors and state your reason. Add any missing resonance forms
NH2 NH2
H2 H2 CH3 C C NH2 CH3 C C NH2
1 Question II
1. Predict the products of the following acid-base reaction. Using curve arrows to show how they exchange H+.
O
HO C OH + 2 OH
2. Label each reactant as Lewis acid and Lewis base. Then using curve arrow to show how the products are made.
Cl
AlCl Al CH3OCH3 + 3 Cl O CH3
Cl CH
3. Predict whether reactant or products are favored at equilibrium. Why?
+ NH CH3CH2OH + NH2 CH3CH3O 3 pKa = 36 pKa = 16.0
4. Without using pKa values, explain which compound is a stronger acid. Hint: Draw and explain the stability of conjugate base
2 CH CH or CH NO a. 3 3 3 2
SH OH
or b. Question 3
1. Using bond-line to draw the following molecules
a. sec-butyl amine b. propylcyclopentane
b. cis-1-bromo-3-cyclohexane d. 1,5-hexanediol
5. Give a systematic name for each of the following molecule
3 OH a. b. Br
c. Cl d.
Question 4:
6. Using Newman projections to draw the all eclipsed and staggered of 2,3-dimethyl-2-pentanol. Label Anti and gauche then rank them in the order of stability. Draw an energy diagram
OH
Br
4 7. Draw the most stable conformer of trans-1-ethyl-2-isopropylcylohexane
8. Draw the chair conformation and the result of ring flips. Determine the most stable conformer and explain your reason. Don’t forget to label all carbon atoms
1
5 3
5 Question 5:
1. Rank these molecules or compound according to their boiling point, starting from the highest
OF2, LiF, F2 and CH3OH
Heptanes, 3-hexanol, n-hexylamine, 2-pentanol
2. Show hydrogen bonding between methanol and water in two different ways then identify the H-bond donor and H-bond acceptor
6