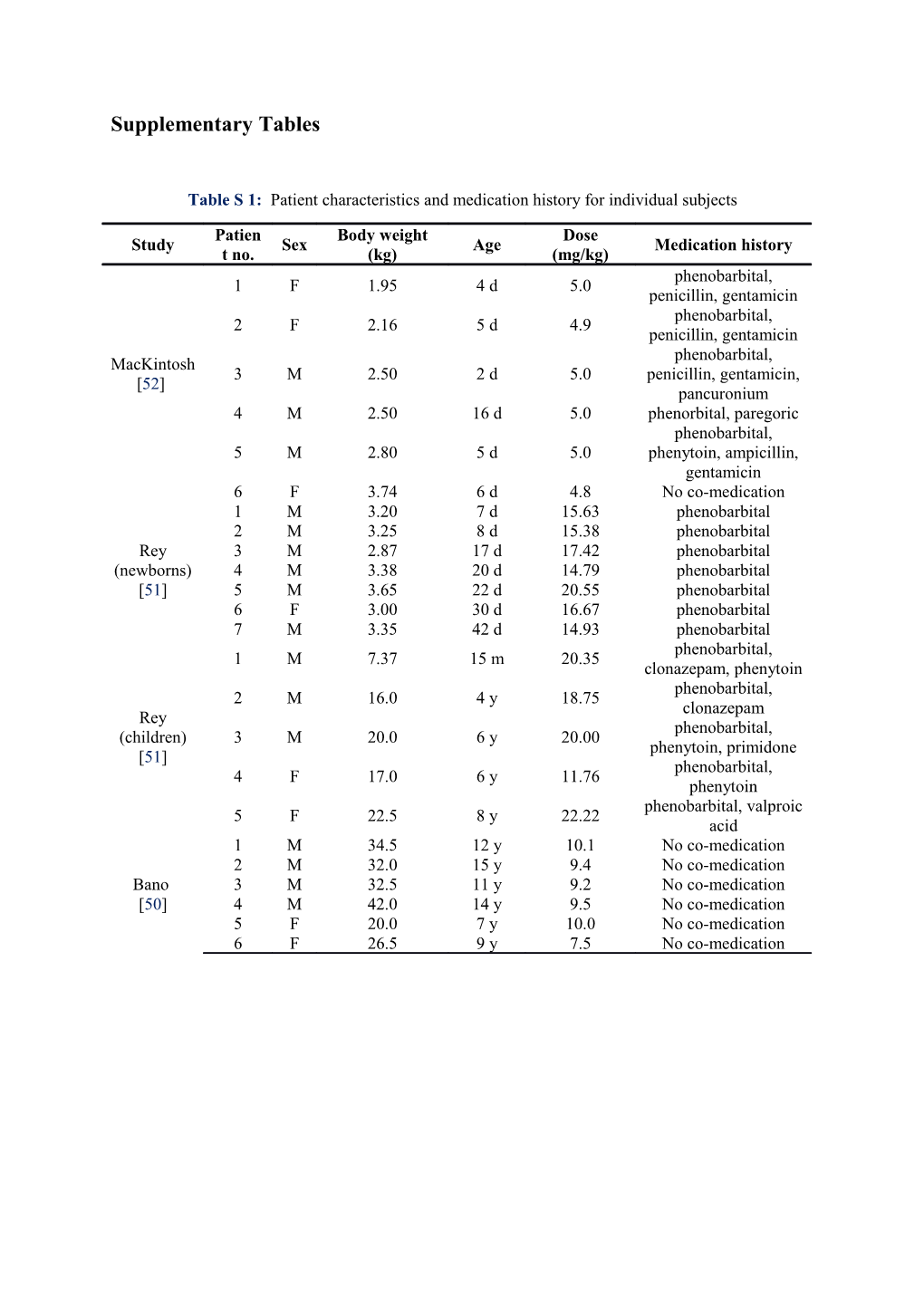
Supplementary Tables
Table S 1: Patient characteristics and medication history for individual subjects
Patien Body weight Dose Study Sex Age Medication history t no. (kg) (mg/kg) phenobarbital, 1 F 1.95 4 d 5.0 penicillin, gentamicin phenobarbital, 2 F 2.16 5 d 4.9 penicillin, gentamicin phenobarbital, MacKintosh 3 M 2.50 2 d 5.0 penicillin, gentamicin, [52] pancuronium 4 M 2.50 16 d 5.0 phenorbital, paregoric phenobarbital, 5 M 2.80 5 d 5.0 phenytoin, ampicillin, gentamicin 6 F 3.74 6 d 4.8 No co-medication 1 M 3.20 7 d 15.63 phenobarbital 2 M 3.25 8 d 15.38 phenobarbital Rey 3 M 2.87 17 d 17.42 phenobarbital (newborns) 4 M 3.38 20 d 14.79 phenobarbital [51] 5 M 3.65 22 d 20.55 phenobarbital 6 F 3.00 30 d 16.67 phenobarbital 7 M 3.35 42 d 14.93 phenobarbital phenobarbital, 1 M 7.37 15 m 20.35 clonazepam, phenytoin phenobarbital, 2 M 16.0 4 y 18.75 clonazepam Rey phenobarbital, (children) 3 M 20.0 6 y 20.00 phenytoin, primidone [51] phenobarbital, 4 F 17.0 6 y 11.76 phenytoin phenobarbital, valproic 5 F 22.5 8 y 22.22 acid 1 M 34.5 12 y 10.1 No co-medication 2 M 32.0 15 y 9.4 No co-medication Bano 3 M 32.5 11 y 9.2 No co-medication [50] 4 M 42.0 14 y 9.5 No co-medication 5 F 20.0 7 y 10.0 No co-medication 6 F 26.5 9 y 7.5 No co-medication Table S 2: Data characteristics of empirical clearance models
Parameter Reith [75] Gray [78] Yukawa [77] Iribarnegaray [76]
No. of patients 91 72 466 201
No. of observations 946 118 1010 387 Mean number of observations per subject 4 (1-40) 1.6 1.9 1.9 (1-11) (range) Mean age 18.14 y 8.7 y 15 y 9.5 y (range) (0.7-37.2) (2.3-16.3) (0.3-72.9) (1-14) Mean body weight 63.57 kg 29.5 kg 41.3 kg 35 kg (range) (9.8-106) (9-80) (4.5-90) (9-78) Mean dose 9.13 mg/kg/d 14.4 mg/kg/d 12.8 mg/kg NA (range) (1.5-26.8) (2.4-35.3) VPA 10% a 26% a 17.5% a 9.45%b
PHT 1.2% a 10% a NA NA
PB 1.4% a 8% a 14.3% a 7.96%b
Two or more AED NA 1% a 40.9% a NA VPA valproic acid; PHT phenytoin; PB phenobarbital, AED antiepileptic drugs, NA not available. Information about co-medication in terms of percentage and samples per subject were either directly extracted or calculated based on information about patient numbers a Percentage of observations from patients receiving co-medication b Percentage of patients receiving co-medication Table S 3: Parameter ranges selected for sensitivity analyses
Parameter Lower bound Baseline value Upper bound Stomach transit time 0.25 h 0.5 h 1 h Stomach volume 5 mL 25 mL 50 mL Fraction of SI fluid 0.5x baseline value 40% 2x baseline value volume SI length 0.5x baseline value Age-dependent 2x baseline value SI radius 0.5 cm 1 cm 2 cm SI transit time 0.5x baseline value Age-dependent 2x baseline value Bile salt solubilization 420.5 4205 42050 ratio Aqueous solubility 0.012 mg/mL 0.12 mg/mL 1.2 mg/mL Permeability 2.15 x 10-4 cm/s 4.3 x 10-4 cm/s 8.6 x 10-4 cm/s Drug particle radius 2.5µm 25 μm 250 µm
Dose volume 5 mL 25 mL 50 mL Dose 0.1x of baseline value As detailed in study 10x baseline value report Table S 4: Composition of simulated gastric media [11, 81]
FaSSGF Pn-FaSSGF FeSSGF Pnc-FeSSGF Sodium chloride (mM) 34.2 34.2 237.02 100.35 Acetic acid (mM) - - 17.12 7.25 Sodium acetate (mM) - - 29.75 64.65 Sodium taurocholate (µM) 80 20 - - Lecithin (µM) 20 5 - - Pepsin (mg/mL) 0.1 0.015 - - Milk:buffer (v/v) - - 1:1 1:1 pH 1.6 1.6 5 5.7 Osmolarity (mOsm/L) 120.7 120.7 400 240 FaSSGF Adult fasted-state simulated gastric fluid; Pn-FaSSGF Paediatric fasted-state simulated gastric fluid representative of neonates; FeSSGF Adult fed-state simulated gastric fluid; Pnc-FeSSGF Paediatric fed-state simulated gastric fluid representative of neonates prepared using cow’s milk-based formula Table S 5: Composition of simulated intestinal media [11, 81]
P-FaSSIF P-FaSSIF FaSSIF FeSSIF Pnc-FeSSIF 50 % 150 %
Sodium chloride (mM) 105.92 68.62 68.62 203.11 111.73
Maleic acid (mM) - 19.12 19.12 - 55.02
Sodium hydroxide (mM) 10.5 34.8 34.8 101 81.65 Sodium 28.63 - - - - dihydrogenphosphate (mM)
Sodium taurocholate (mM) 3 1.5 4.5 15 2.5
Lecithin (mM) 0.75 0.1 0.3 3.75 0.5
Glycerol monooleate (mM) - - - - 6.65
Sodium oleate (mM) - - - - 1.06
pH 6.5 6.5 6.5 5.0 5.8
Osmolarity (mOsm/L) 270 180 180 635 330
FaSSIF Adult fasted-state simulated intestinal fluid; P-FaSSIF 50% Paediatric fasted-state simulated intestinal fluid formulated with bile salt concentrations corresponding to 50% of adult levels; P-FaSSIF 150% Paediatric fasted-state intestinal fluid formulated with bile salts concentration corresponding to 150% of adult levels; FeSSIF Adult fed-state simulated intestinal fluid; Pnc-FeSSIF Paediatric fed-state simulated intestinal fluid representative of neonates prepared using cow’s milk-based formula Table S 6: Experimentally measured CBZ solubility in simulated gastric and intestinal fluids (n=3, 37°C)
Medium Mean solubility ± SD (µg/mL) Pn-FaSSGF 208.8 ± 3.9 FaSSGF 208.7 ± 19.9 Pnc-FeSSGF 185.9 ± 16.0 FeSSGF 329.6 ± 25.5 P-FaSSIF 50 % 206.3 ± 10.9 P-FaSSIF 150 % 234.9 ± 4.3 FaSSIF 233.6 ± 7.4 Pnc-FeSSIF 245.4 ± 15.8 FeSSIF 343.1 ± 8.9 Table S 7: List of the anatomical, physiological and drug specific parameters important for drug
absorption and the basis for the default values included in the paediatric absorption models in
GastroPlus.
Parameters GastroPlus paediatric models Volume of GI organs Age-specific scaling GI organs blood flows Age-specific scaling Intestinal radius Age-specific scaling Intestinal length Age-specific scaling Intestinal surface area Age-specific scaling Gastric pH No scaling (adult values) Intestinal pH No scaling (adult values) GET No scaling (adult values) SITT Age-specific scaling Fluid secretion volume Age-specific scaling Solubility Unchanged with age Permeability coefficient in the gut wall Unchanged with age
GI gastrointestinal; GET mean gastric emptying time; SITT small intestine transit time Supplementary Figures
Figure S 1: Goodness of fit plots for plasma concentrations and area under the plasma concentration- time curve (AUC) of the CBZ PBPK model for adults after administering CBZ solution, suspension, and IR tablet. Figure S 2: Impact of age on simulated fraction of dose absorbed in fasted state for five different dose levels. Figure S 3: Parameter sensitivity analysis results for (a) stomach transit time, (b) stomach volume, (c) small intestine length, (d) small intestine radius, (e) bile salts solubilisation ratio, (f) permeability, and (g) dose volume. Grey coloured profiles represent data in neonates, black profiles are data in older children; low doses are illustrated as continuous lines and high doses as dotted lines. The baseline values used in the models are shown as a red circle. Figure S 4: Dissolution profiles of CBZ in adult and paediatric biorelevant media. Figure S 5: (a, b) Simulations of a 310 mg dose in a 5-year-old child of 17 kg body weight overlaid with data from Rey [51] (linear and logarithmic scale for plasma concentration, respectively). (c, d) Simulations of a 54 mg dose in a 21 day old newborn infant overlaid with data from Rey [51] (linear and logarithmic scale for plasma concentration, respectively). The lines show simulations achieving a range of fraction absorbed by varying the reference solubility in the models. The systemic clearances used in both models were those which had been found to deliver the best match to the clinical observations as described, namely 1.3 L/h for the child and 0.3 L/h for the neonate. Supplementary Figures – Adult PBPK model
Figure S 6: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving a CBZ solution. Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a, b, c) Levy et al. [34], (d, e, f) Rawlins et al. [16], (g) Sumi et al. [43], (h) Kaneniwa et al. [44].
Figure S 7: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving a CBZ suspension. Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a, b) Zhang et al. [33], (c) Neuvonen et al. [44], (d) Graves et al. [47]. Figure S 8: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving CBZ tablets (Tegretol). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a, b) Levy et al. [34], (c) Rawlins et al. [16], (d, e) Zhang et al. [33], (f, g, h) Gérardin et al. [17]. Figure S 9: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving CBZ tablets (Tegretol). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a) Kaneniwa et al. [40], (b) Sumi et al. [39], (c) Graves et al. [47], (d) Anttila et al. [18], (e) Popovic et al. [48], (f) Wada et al. [46], (g) Hooper et al. [45], (h) Cotter et al. [27]. Figure S 10: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving CBZ tablets (Tegretol). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a) Neuvonen et al. [31], (b) Wong et al. [32], (c) Paxton et al. [41], (d) Riad et al. [42], (e) Jung et al. [43], (f) Johannessen et al. [35], (g) Strandjord et al. [23], (h) Morselli et al. [24]. Figure S 11: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving CBZ tablets (Tegretol), except (af receiving the generic formulation Mazetol). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state, and number of study subjects are shown above the plot. (a) Chan et al. [25], (b) Olling et al. [29], (c) Langguth et al. [27], (d) Cotter et al. [37], (e) Kauko et al. [49], (f) Pynnönen et al. [28], (g, h) Bhatia et al. [30]. Figure S 12: Simulated plasma concentration - time profiles (dashed line) in adult subjects receiving CBZ tablets (Tegretol). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Dose, nutritional state and number of study subjects are shown above the plot. (a) Dam et al. [36], (b) Ferreira et al. [19], (c) Meyer et al. [20], (d) Revankar et al. [21], (e) Kayali et al. [22], (f) Maas et al. [38].