Supplemental Section: A New Approach to High Sensitivity Liquid Chromatography-Mass
Spectrometry of Peptides using Nanoflow Solvent Assisted Inlet Ionization
Wang, B., Inutan, E.D., and Trimpin, S.*
Department of Chemistry, Wayne State University, Detroit, MI 48202, USA
*Correspondence to: S. Trimpin, Department of Chemistry, Wayne State University, Detroit, MI 48202,
USA. Phone: (313) 577-9823. E-mail: [email protected]
600
500
400 y t i s n
e 300 t n I
n o
I 200
100
0 -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 0.4 Distance (mm)
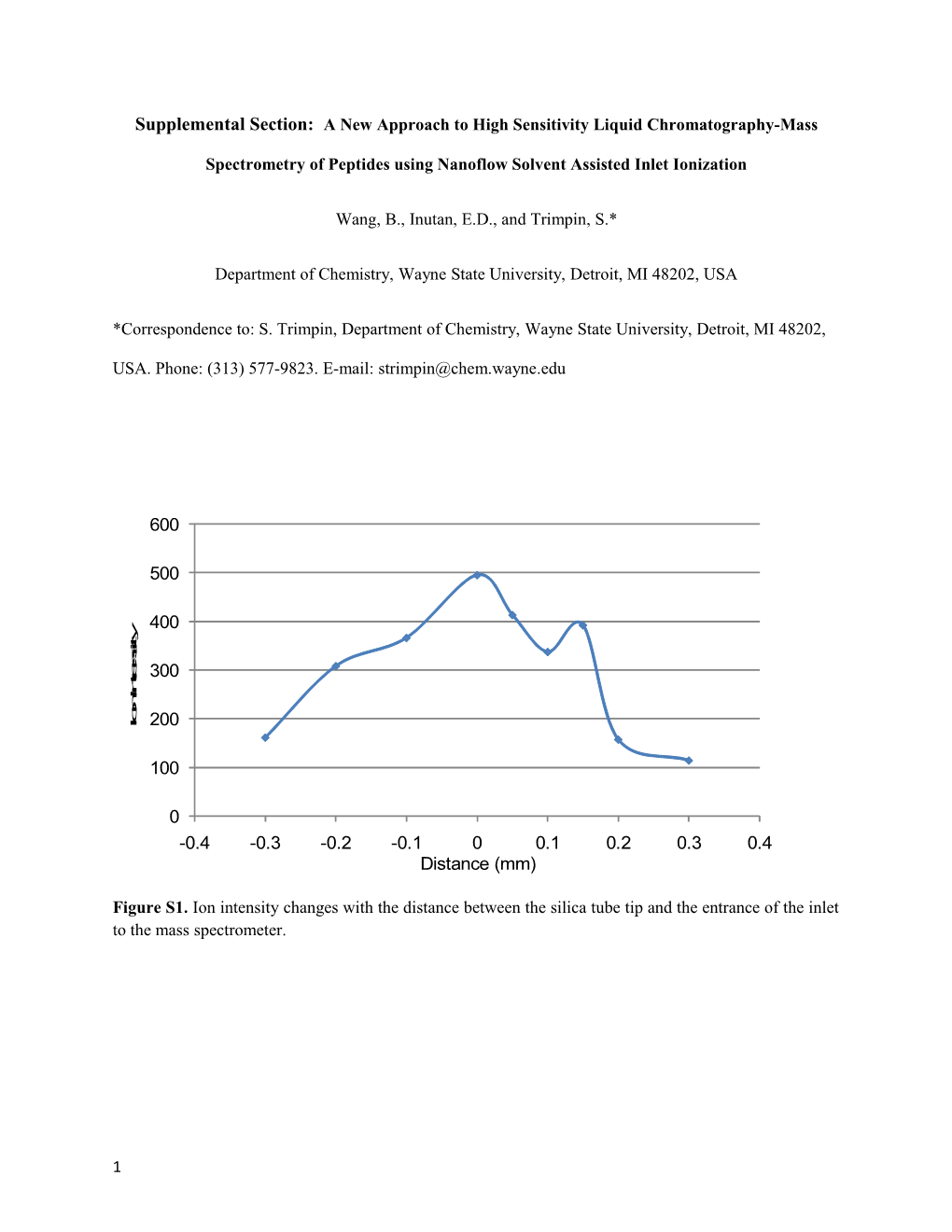
Figure S1. Ion intensity changes with the distance between the silica tube tip and the entrance of the inlet to the mass spectrometer.
1 Mechanistic Aspects: Laserspray ionization (LSII), potentially useful for imaging at high spatial resolution,1-23456 is a subset of matrix assisted inlet ionizatrion (MAII)7 in which a laser is used to transfer the matrix/analyte mixture to the mass spectrometer inlet where ions are produced in the heated pressure drop region having similar charge states to ESI. We hypothesize that ion formation is vacuum and thermal assisted.4,6,8-910 SAII11,12 is similar to the other inlet ionization methods of LSII1-6, and MAII7 in which any means of introducing matrix/analyte into the heated inlet produces ions similar to ESI. In the case of SAII11, where the matrix is a solvent, it can be shown (Figure S2) that ions are not produced by a sonic spray mechanism as is evident by the requirement that the inlet be heated to produce ions: heat is not a requirement for sonic spray.13,14 There are also significant differences between inlet ionization with a solvent and thermospray ionization15 in addition to the high charge states, low flow rates and the high sensitivity achievable with SAII, as was recently pointed out.12
+9 A.1 952.52+8 B.1 +7 100 1071.44 2.64E2 100 1224.36 1.03E2 +10 e e c 857.44 c n n a a d +7 d +6 n n +5 u +11 1224.19 u 1428.03 b b
A 1713.54 779.60 A
e e v v +8 i +6 i
t 675.68 t a a l 1428.12 l +91071.27 e +5 e 637.26 R 1713.71 R +10952.35 857.44 0 0 A.2 +7 B.2 +7 1224.36 1224.44 100 +6 7.62E2 100 1.27E4 +8 1428.28 e 1071.69 e +6 c c +8 n n 1428.28
a +9 a 1071.69 d d n 952.77 n +9 u u +5 b b +10 952.69 A A 1713.62
e +10 e 857.60 v +5 v i i +11 t +12 t a 857.60 a l +11 1713.46 l 779.68 e e +12 R 779.60 R 714.68 714.93 0 0 600 800 1000 1200 1400 1600 1800 600 800 1000 1200 1400 1600 1800 m/z m/z
Figure S2. SAII-MS of ubiquitin. The liquid solvent/analyte droplet is placed with a pipette tip (10 uL plastic tip) vertically just outside of the mass spectrometer inlet and slowly pipetted off. The droplets formed are vacuum drawn into the inlet of the mass spectrometer where inlet ionization of the analyte occurs. Solutions of 5 pmol ubiquitin (10 µL) were used. Depending on the solution conditions and inlet
2 temperature the abundance of the highly charged ubiquitin ions varies; less volatile solvent conditions require higher inlet temperatures: (A) ubiquitin in 100% water solution and (1) 300 °C and (2) 450 °C and (B) ubiquitin in 50:50 ACN/H2O in 1% formic acid solution and (1) 50 °C and (2) 300 °C applied on the inlet of the mass spectrometer. In pure water, Na+ adducts are in high abundance lowering the overall intensity of any single peak. Addition of formic acid as shown in B almost eliminates the Na+ adducts.
3 Figure S3. Picture showing the setup for LC-nSAII: Front view of Waters NanoAcquity liquid chromatograph, Thermo LTQ-Velos mass spectrometer and, here, an automated x,y,z-stage for alignment of tip (Figure 1a) and the “picotip” extension (Figure 1b) of the fused silica capillary tube of the LC column placed about 0.1 mm out of the orifice inlet entrance of the mass spectrometer.
4 582 A. 100 653 571 e c
n 734 a d n
u 820 b A 789 746 e v
i 740 700 t a l e
R 758 1014 569
0 8 10 12 14 16 18 20 22 24 26 Time (min) 653 B. 100 582 e c
n 746 a d
n 820 u
b 571 A
e v i 734 t a l 740 700 e R 789 758 1014
0 4 6 8 10 12 14 16 18 20 22 24 Time (min)
Figure S4. LC-SAII-MS of 100 fmol µL-1 BSA tryptic digest acquired on the Thermo LTQ Velos mass spectrometer. (A) The base peak chromatogram of 1 µL injection at 0.8 µL min -1 flow rate; (B) the base peak chromatogram of 1 µL injection at 1.2 µL min-1 flow rate. The m/z of the most intense peak from the mass spectra of each LC chromatographic peak is labeled at the top of the peak. The mass range for MS data acquisition is 555-1600.
5 507 A. 100 582
653 e c n a d n
u 700 b 746 A
e v
i 734 t a l 789 863 e
R 758 820 740 527 569
0 8 10 12 14 16 18 20 22 24 26 507.75 Time (min) 100 B. [M+2H]2+ e c n a d n u b A
e + v
i [M+H] t a l 1014.68 e R
0 500 700 900 1100 1300 1500 m/z
Figure S5. A 50 min LC run injecting 1 µL of a 100 fmol µL-1 BSA tryptic digest solution analyzed by LC-nSAII-MS at a mobile phase flow of 800 nL min-1. (A) A section of the base peak chromatogram. The start trigger for the mass spectral acquisition was the same as for the LC so that the inject time is included in the X-axis. The most intense m/z obtained on the mass spectra for each chromatographic peak is labeled. (B) Mass spectrum of the chromatographic peak at 17.8 min.
6 746 A. 100 863
734 498 653 700
% 722 722 225 223 569 848
0 10.00 14.00 18.00 22.00 26.00 30.00 34.00 Time (min) 863 100 B. 746 582 734 700 722 740 874653 %
722 225
0 10.00 14.00 18.00 22.00 26.00 30.00 34.00 Time (min)
Figure S6. Base beak chromatogram of LC-nESI-MS of 100 fmol µL-1 BSA tryptic digest on Waters SYNAPT G2 mass spectrometer. The skimmer temperature is held at 150 ºC. (A) The base peak chromatogram of 1 µL injection at 800 nL min-1 flow rate; (B) the base peak chromatogram of 1 µL injection at 400 nL min-1 flow rate. Peaks are labeled with m/z values.
7 References
8 1. Trimpin, S., Herath, T.N., Inutan, E.D., Cernat, S.A., Wager-Miller, J., Mackie, K., Walker, J.M.: Field- free Transmission Geometry Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization for Rapid Analysis of Unadulterated Tissue Samples. Rapid Commun. Mass Spectrom. 23, 3023-3027 (2009) 2. Inutan, E.D., Richards, A.L., Wager-Miller, J., Mackie, K., McEwen, C.N., Trimpin, S.: Laserspray Ionization-A New Method for Protein Analysis Directly from Tissue at Atmospheric Pressure and with Ultra-High Mass Resolution and Electron Transfer Dissociation Sequencing. Mol. Cell. Proteomic 10, 1-8 (2011) 3. Trimpin, S., Inutan, E.D., Herath, T.N. McEwen, C.N.: A Matrix-assisted Laser Desorption/Ionization Mass Spectrometry Method for Selectively Producing Either Singly or Multiply Charged Molecular Ions. Anal. Chem. 82, 11-15 (2010) 4. Inutan, E.D., Trimpin, S.: Laserspray Ionization (LSI) Ion Mobility Spectrometry (IMS) Mass Spectrometry (MS). J. Am. Soc. Mass Spectrom. 21, 1260-1264 (2010) 5. Richards, A.L., Lietz, C.B., Trimpin, S.: Imaging Mass Spectrometry in Transmission Geometry. Rapid Commun. Mass Spectrom. 25, 815-829 (2011) 6. Trimpin, S., Inutan, E.D., Herath, T.N., McEwen, C.N.: Laserspray Ionization-A New AP-MALDI Method for Producing Highly Charged Gas-Phase Ions of Peptides and Proteins Directly from Solid Solutions. Mol. Cell. Proteomics 9, 362-367 (2010) 7. McEwen, C.N., Pagnotti, V., Inutan, E.D., Trimpin, S.: A New Paradigm in Ionization: Multiply Charged Ion Formation from a Solid Matrix without a Laser or Voltage. Anal. Chem. 82, 9164-9168 (2010) 8. McEwen, C.N., Trimpin, S.: An Alternative Ionization Paradigm for Atmospheric Pressure Mass Spectrometry: Flying Elephants from Trojan Horses, Int. J. Mass Spectrom. 300, 167-172 (2011) 9. Trimpin, S., Jing, L., Wang, B., Ren, Y., Lietz, C.B., Richards, A.L., Lingenfelter, S., Marshall, D.D., Inutan, E.D., Ionization Inlet and at Vacuum: New Aromatic and Non Aromatic Matrix Compounds Producing Highly Charged Peptide and Protein Ions, J. Am. Soc. Mass Spectrom. under revision. 10. Trimpin, S., Wang, B., Inutan, E.D., Li, J., Lietz, C.B., Pagnotti, V.S., Harron, A.F., Sardelis, D., McEwen, C.N. A Unified Mechanism for Ionization of Nonvolatile Compounds in Mass Spectrometry: Lessons from MALDI and LSI, J. Am. Soc. Mass Spectrom. submitted. 11. Pagnotti, V.S., Chubatyi, N.D., McEwen, C.N.: Solvent Assisted Inlet Ionization: An Ultrasensitive New Liquid Introduction Ionization Method for Mass Spectrometry. Anal. Chem. 83, 3981-3985 (2011) 12. Pagnotti, V.S., Inutan, E.D., Marshall D.D., McEwen, C.N., Trimpin, S.: Inlet Ionization: A New Highly Sensitive Approach for Liquid Chromatography-Mass Spectrometry of Small and Large Molecules. Anal. Chem. 83, 7591-7594 (2011) 13 . Hirabayashi, A., Sakairi, M., Koizumi, H.: Sonic Spray Ionization method for atmospheric-pressure ionization mass-spectrometry. Anal. Chem. 66, 4557-4559 (1994) 14 . Hirabayashi, A., Hirabayashi, Y., Sakairi, M, Koizumi, H.: Multiply-charged ion formation by sonic spray, Rapid Commun. Mass Spectrom. 10, 1703-1705 (1996) 15. Vestal, M. L.: Ionization techniques for nonvolatile molecules. Mass Spectrom. Rev. 2, 447-480 (1983)